Elementary Subatomic Particles
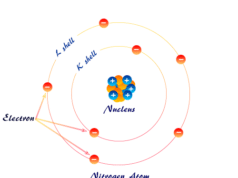
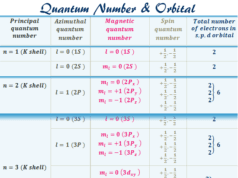
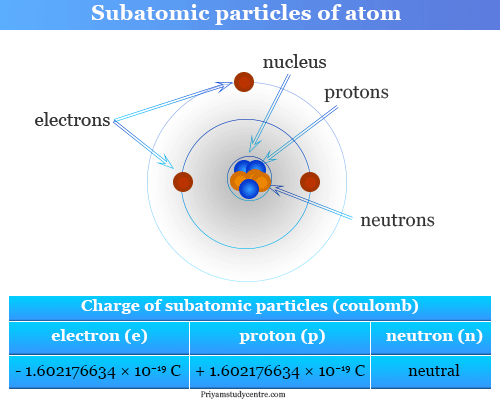
Elementary particles or subatomic particles like electrons, protons, and neutrons in physics or chemistry were discovered by Scientists Thomson, Golstine, and Chanweak. These three particles are the fundamental part of an atom in the periodic table of chemical elements. Fundamental particles like protons and neutrons are located in the positive charge nucleus. Electrons are the extranuclear subatomic part of an atom that carries a negative charge. Present-day, the atomic structure, properties, and characteristics or types of chemical bonding in learning chemistry or physics are better described by the orbital energy level and electronic configuration of the elementary subatomic electron particles. The smallest discovered elementary subatomic particle is the quark, the basic building block of hadrons.
Discovery of Subatomic Particles
The idea about the different types of subatomic particles resulted from Faraday’s famous experiment (1837) or electrolysis of salt or acids bases by the passage of electric current.
Faraday establishes the quantitative relations between the amount of substance and the quantity of electricity. He suggested the electric current carries charged elementary particles or ions in the solution.
The standard model of different types of elementary subatomic particles was finely developed from the ionization of gases.
Cathode Ray Tube Experiment
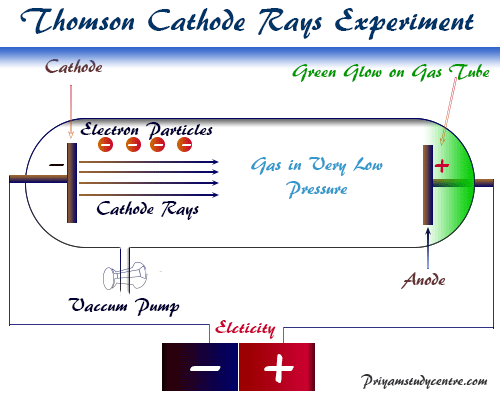
JJ Thomson cathode ray discharge tube experiment suggested the atoms of periodic table chemical elements contain negatively charged elementary particles. He suggested that gases at low pressures are subjected to high potential from various luminous effects.
The pressure is quite low (0.01 mm), the tube remains dark (Crooks dark space) and the rays contain subatomic particles or cathode rays. It traveled from cathode to anode with definite properties.
Properties of Cathode Rays
- Production of fluorescence on the opposite wall where the rays impinge.
- The rays travel in straight lines confirmed by the shadows of an object placed on its path.
- The cathode rays are defective from the path they travel by electric or magnetic field. Here, the direction of deflection suggested that the cathode ray carries the negative charge fundamental elementary particle.
- When these electromagnetic rays impinge on the crystalline solid metal targets placed on their path x-ray is produced.
Charge of an Electron
An electron is a negatively charged elementary subatomic particle of an atom. Hence the negative charge carries by an electron,
e = − 4.8 × 10−10 esu
= − 1.602176634 × 10−19 coulomb (C)
Mass of an Electron
Mass of an electron = m and charge = e. Charge by mass (e/m) ratio for electron = 1.76 × 108 coulomb/g.
From the above formula, the mass of an electron particle,
me = (1.602176634 × 10−19)/(1.76 × 108) g
= 9.1093837015 × 10−28 g
= 9.1093837015 × 10−31 kg
The mass of electrons is very small in comparison to neutrons or protons. It is only 1/1836 mass of a proton. Therefore, we can not consider the mass of an electron for calculating the mass number of an atom.
How to Calculate the Charge of an Electron?
Faraday’s law of electrolysis of silver nitrate is used for the determination of the charge of the subatomic particles electron in physics or chemistry in science. He used metallic silver as a reducing agent at the cathode decreasing the oxidation number or gaining one electron.
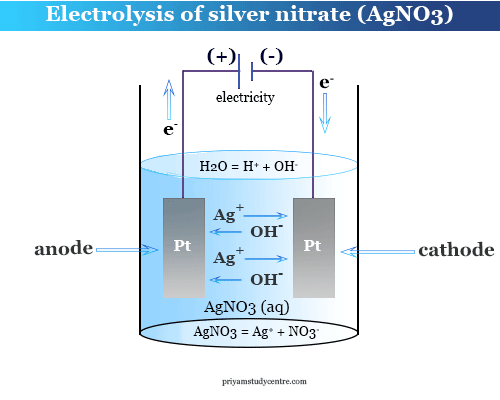
The Avogadro number of electrons produced 1 mole of silver at the electrode from this redox reaction. At the same time, 1 mole of electrons is removed from the anode, and 1 mole of nitrate ions is discharged.
According to Faraday’s law, 96500 coulombs of electrical energy are required for the production of 1 gm equivalent silver at the electrode. Therefore, the charge carried by subatomic particle or electron,
e = (96,500 coulomb mol−1)/(6.023 × 1023 mol−1)
= 1.60 × 10−19 coulomb
Proton Particles
Subatomic electron particle contributes negligibly to the mass of the atom but an atom is electrically neutral. Hence the nucleus of an atom must contain another subatomic particle called a proton. It carries both the mass and positive charge of an atom.
Goldstein added a new feature to the discharge tubes by using holes in the cathode. With this modification, he observed that there appeared not only cathode rays but also a beam of positively charged particles traveling from the anode to the cathode.
Some of the positively charged subatomic elementary particles pass through the hole in the cathode and produce a spot on the far end of the discharge tube.
Properties of Proton
Nature and location of subatomic particles proton found by Thomson in science.
- On deflection by a magnetic and electric field, the positive ray beam produced a large diffuse spot on the tube.
- The e/m ratio and velocity of these elementary particles are not the same as electrons. For example, the hydrogen atom is the simplest discovery elementary particle that carries one electron and proton without any neutron.
- The nucleus of the hydrogen atom contains an elementary particle proton with a unit positive charge.
Charge of Proton
It is the positively charged elementary subatomic particle of an atom. The charge of the proton is equal to the magnitude of the electron. Hence the charge of positive charge or proton,
= + 4.8 × 10−10 esu
= + 1.602176634 × 10−19 coulomb (C)
Mass of Proton
Mass of proton = m and charge = + e. Charge by mass ratio (e/m) for proton = 9.3 × 104 coulomb/gram.
Hence the mass of the proton,
mp = 1.67262192369 × 10−27 kg
= 1.67262192369 × 10−24 g
Who Discovered Neutron?
The entire mass of an atom concentrated in the nucleus because the weight of electrons is negligible. Atomic number and mass number of hydrogen = 1. Therefore, protons alone account for the total mass of the hydrogen atom. But except for hydrogen, the proton alone cannot account for the total mass of the nucleus.
For example, the helium atom is 4 times as heavy as an atom of hydrogen, hence the helium nucleus must be 4 times heavier than the subatomic particle proton.
To solve these facts Chadwick discovered a new fundamental elementary subatomic particle neutron found in the nucleus of an atom.
Let the mass number of an atom = A, nuclear charge, or the number of protons of the atom = Z. Therefore, (A − Z) shortfall of the mass number found by other subatomic neutron particles.
How to Find the Number of Neutrons?
Rutherford suggested this shortfall must be made up by another elementary particle. Rutherford named this elementary atomic particle in advance, a neutron. It is electrically neutral, and its mass is equal to that of the proton. The glory of the discovery of elementary particles neutron is given to the scientist Chadwick, one of Rutherford’s students.
The mass number and the atomic number of oxygen 16 and 8 respectively suggested that the atomic nucleus of oxygen contains 8 protons or 8 neutrons on the nucleus.
Isotopes Examples
The atoms of an element that contain the same number of subatomic proton particles but varying numbers of neutrons inside the nucleus are called isotopes. Therefore, isotopes contain atoms of the same element but carry different types of mass numbers.
- Protium, deuterium, and tritium are the list of hydrogen isotopes that carry zero, one, and two elementary neutron particles with mass numbers, 1, 2, and 3 respectively.
- The isotopes of oxygen (O-16 and O-17) contain eight and nine subatomic neutron particles in the nucleus.
- The radioactive isotopes of uranium, thorium, and lead are the other examples where the number of neutrons inside the nucleus is different.