Alpha Beta Gamma Rays Properties
Alpha, beta, gamma rays radiation in radioactivity is the spontaneous radioactive decay of atomic particles in the form of energy or electromagnetic radiation. Among these three, alpha and beta particles are deflected by the magnetic and electric field but gamma rays are made by electromagnetic waves with very small wavelengths. The charge, mass, velocity, and penetrating properties of alpha, beta, and gamma particles, or rays are different. The emission of alpha-beta and gamma rays from different radiation sources is independent of pressure, temperature, pH scale, and specific heat of the surroundings. This is due to the activation energy for radioactive radiation being zero. The radioactive decay of alpha, beta, and gamma rays follows the first-order chemical kinetics and the half-life is independent of the amount of the sample.
Alpha Particles
Alpha particles or alpha rays (symbol α) consist of a stream of positively charged particles that carry +2 charge and mass number 4. Rutherford showed that the alpha particle is identical to the nuclei of the helium atom. Therefore, the alpha particle is a doubly charged helium ion (He+2) with atomic number 2 and mass number 4.
Properties of Alpha Particles
The properties of alpha particles are based on several experimental results. The charge/mass ratio (e/m) of alpha particles was determined by the study of the Thomson electron deflection experiment. The e/m of the alpha particle is almost half of the e/m of the proton. Therefore, it carries two units of charge and its mass must be four times larger than that of the proton.
| Alpha particles | ||
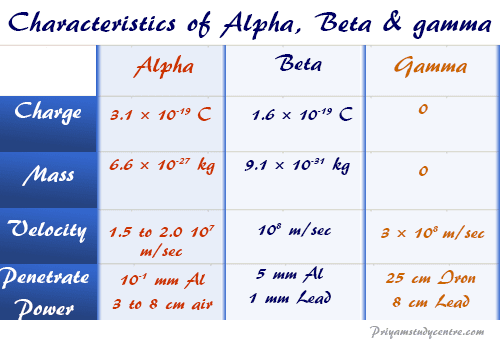
| Charge | Mass | Velocity |
| + 3.1 × 10−19 C | 6.6 × 10−27 kg | 1.5 to 2.0 × 107 m/s |
Alpha Particle Emission
When an alpha particle is ejected from within the nucleus of an atom, the mother element loses two units of atomic number and four units of mass number. For example, the emission of an alpha particle from the uranium nucleus (atomic number 92, mass number 238), the nucleus of the daughter element will have an atomic number 90 and mass number 234.
Checking the periodic table we concluded that the daughter element is an isotope of thorium.
92U238 → 90Th234 + 2He4 (alpha-ray)
What is a Beta Particle?
Beta particle also called beta ray or beta radiation (symbol β) is the stream of negatively charged particles. From the study of deflection in a magnetic or electric field, the properties of a beta particle are identical to those of an electron. Therefore, the e/m values of beta ray beta ray or electron = 1.77 × 108 coulombs/g.
| Beta particle | ||
| Charge | Mass | Velocity |
| – 1.6 × 10−19 C | 9.1 × 10−31 kg | 108 m/s |
Emission of Beta Particle
The emission of a beta particle (mass number 0 and charge 1) results in the transformation of a neutron into a proton. When a beta particle is emitted from the nucleus, the daughter nucleus has an atomic number one unit greater than that of the mother nucleus. Spontaneous beta decay was observed in the transformation of 90Th234 to 91Pa234.
| Beta decay equation |
| 0n1 → 1H1 + −1e0 |
| 90Th234 → 91Pa234 + −1e0 |
| 6C14 → 7N14 + −1e0 |
| 53I133 → 54Xe133 + −1e0 |
Difference Between Beta Particle and Electron
Although beta particles and electrons are identical in their electrical nature and charge/mass ratio. The fundamental difference between them,
- The emission of an electron converts a neutral atom into a positively charged ion but leaves the nucleus undisturbed.
- But the emission of a beta particle changes the composition of the nucleus and produces an atom of the next higher atomic number.
What are Gamma Rays?
Gamma rays (symbol γ) consist of electromagnetic radiation of a very short wavelength (λ ∼ 0.004 – 4 Å). These are high-energy photons. The emission of gamma rays accompanies all nuclear reactions. Gamma rays do not carry charge or mass. Hence the radiation of gamma rays cannot change the mass number or atomic number of mother elements. Commonly, the emission of gamma rays is not shown in a nuclear reaction.
How are Gamma Rays Produced?
During the nuclear reactions, there occurs a change in the energy of the nucleus due to the emission of alpha or beta particles. The unstable, excited nucleus stabilizes by the emission of high energy electromagnetic radiation. These high energy electromagnetic radiations are called gamma rays. After the emission of gamma rays, the nucleus drops down to a lower and more stable energy state.
Properties of gamma rays
- The gamma rays have much greater penetrating power than alpha or beta rays. It can be detected even after passing through 30 cm of iron.
- Gamma rays are not deviated by an electric or magnetic field. Therefore, they do not carry any charge.
- Gamma rays are electromagnetic waves of very short wavelengths ranging between 4 Å to 0.04 Å.
- They are comparable to X-rays and differ from the latter only in respect of their origin.
- The γ-rays affect photographic plates and are capable of ionizing gases.
Positron Emission
Curies and Rutherford discover another mode of nuclear transformation rather than alpha, beta, or gamma rays. This involves the ejection of a positron from within the nucleus.
The emission is made by the conversion of a proton into a neutron.
1H1 (proton) → 0n1 (neutron) + +1e0 (positron)
The emission of positron lowers the atomic number by one unit but leaves the mass number unchanged.
51Sb120 → 50Sn120 + +1e0 (positron)
What is Neutrino?
A neutrino is a subatomic particle formed by the ejection of electrons from within the nucleus with a very small mass. The mass of the neutrino = 0.00002 with respect to the oxygen scale. The ejection of an electron from within the nucleus should be represented,
Neutron → proton + electron + neutrino
Properties of Neutrino
According to the principle of conservation of energy or angular momentum, breaking down a neutron into a proton and a beta particle creates a problem. Particles like neutron, proton, and electron have the spin angular momentum = ± h/4π each.
If we see the nuclear reaction,
0n1 → 1H1 + −1e0
The equation balance with respect to change but does not balance with respect to angular momentum. If the angular momentum of the proton and electron is equal to + h/4π each, they exceed the angular momentum of the neutron. Pauli postulated that along with the emission of beta particles another tiny neutral particle namely neutrino is also ejected.
Nuclear Reaction and Chemical Reaction
What is Chemical Reaction?
Chemical reactions involve some loss, gain, or overlap of an outer orbital electron to form a chemical bond. It can be changing the oxidation number or state of the reactant and product atoms. Such reactions cannot alter the composition of the nuclei. Therefore, the atomic number of the reactant elements remains unchanged after a chemical reaction.
What is Nuclear Reaction?
The nuclear reaction involves the radiation of alpha, beta, or gamma particles or rays from inside the nucleus which changes the atomic number of participating elements. In some artificially induced nuclear reactions, the neutron is absorbed by the target nucleus to produce an isotope. Therefore, the nuclear reaction leads to the birth of other elements or produces an isotope of the parent element.
Difference Between Nuclear and Chemical Reactions
Nuclear reactions are different from chemical reactions in the following respects,
- Nuclear reactions change the composition of nuclei but chemical reactions cannot alter the composition of nuclei.
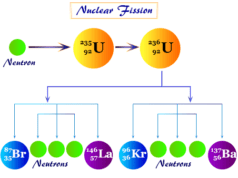
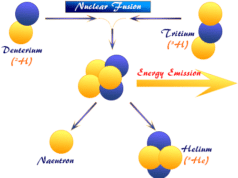
- Nuclear reactions are accompanied by nuclear energy changes by nuclear fusion or nuclear fission reactions which far exceed the chemical energy changes like redox reactions. The energy evolved in the radioactive radiation decay of 1g radium is 5000 times larger than the energy released when 1g radium combines with chlorine.
- The nuclear reactivity of a radioactive element is independent of its state of chemical combination.
- The chemical reactions are dependent on external conditions such as temperature, pressure, pH scale, etc. but nuclear reactions are not.
- Different isotopes of the same element show identical chemical properties since they possess the same electronic configuration. But radioactive isotopes after the emission of alpha, beta, and gamma rays possess different nuclear properties.