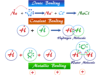
Hydrogen Peroxide (H2O2)
Hydrogen peroxide molecule has molecular formula H2O2 having an interesting skew structural unit. It is used widely in industrial solutions and antiseptic or mouth cleaner in medicine. Due to its high oxidizing properties, it is used as a bleaching agent. A liquid solution of hydrogen peroxide uses in textiles work, paper, pump, lather, and oil industry in our everyday life. In pure form, it is a pale blue liquid that deprotonates to form water and oxygen. Hydrogen peroxide is mostly prepared by the electrolysis of concentrated sulfuric acid by the vacuum distillation process. In hydrogen peroxide structure, two O-H planes lie perpendicular to each other but the solid structural formula is slightly modified by intermolecular hydrogen bonding.
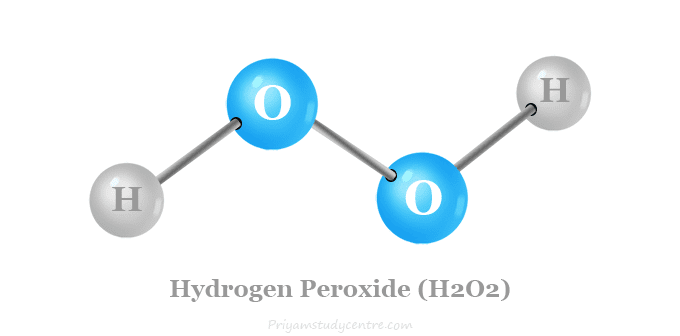
Structure of H2O2
The valence shell electronic configuration of oxygen shows that pz-orbitals oxygen conation a single electron in each orbital in hydrogen peroxide molecule.
Oxygen forms O-O linkages by using the pz-orbitals of the two oxygen atoms. Two oxygen atoms form another covalent bonding with the hydrogen atom. In the H2O2 structure, two O-H planes lie perpendicular to each other but the solid structural formula is slightly modified by intermolecular hydrogen bonding in the molecule.
How to Make Hydrogen Peroxide?
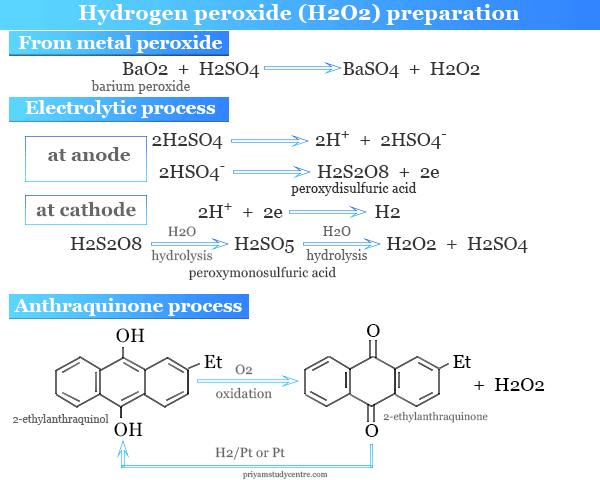
From Metal Peroxide
It may be made in the laboratory by reacting dilute sulfuric acid with crystalline solid barium peroxide (BaO2, 8H2O).
BaO + H2SO4 → BaSO4 + H2O2
Electrolytic Process
- Hydrogen peroxide is made by electrolysis of 50 percent sulfuric acid followed by careful vacuum distillation.
- Peroxydisulfuric acid (H2S2O8) is formed first.
- It is hydrolyzed to produce peroxymonosulfuric acid ( H2SO5).
- It is further hydrolyzed to form H2O2.
Anthraquinone Process
Industrially, hydrogen peroxide can be made by aerial oxidation of 2-ethylanthraquinol in a mixed solvent. Alcohol or ester is used for dissolving quinol and alkyl benzene is used for dissolving quinone.
H2O2 is formed from time to time by shaking with water. The quinone is reduced back to the quinol by catalytic hydrogenation.
From Isopropanol
Hydrogen peroxide is now manufactured during the oxidation of isopropanol liquid or vapour under high pressure (15 to 20 atm) at about 100 °C. It can be separated by fractional distillation.
Chemical Properties
Pure hydrogen peroxide molecule is an extremely pale blue liquid. It shows both oxidizing and reducing properties in acidic or basic mediums.
The oxidation number of oxygen in H2O2 is −1 (normal oxidation number of oxygen = −2).
| Properties of hydrogen peroxide | |
| chemical formula | H2O2 |
| melting point | −0.43 °C |
| boiling point | 150 °C |
| solubility in water | miscible |
| density | 1.450 gm/cm3 (pure) |
| acidity (pKa) | 11.75 |
| Heat capacity | 1.267 J g−1 K−1 (gas) 2.619 J g−1 K−1 (liquid) |
| dipole moment | 2.26 D |
The heat of formation of liquid H2O2 = −187 kJ mol−1. Therefore, it is unstable due to thermodynamics decomposition,
H2O2 (l) → H2O (l) + [O]
At the ordinary temperature or absence of a chemical catalyst such as silver or platinum, the decomposition is very slow.
The decomposition may be explosive at high temperatures. Therefore, it is kept preferably in polythene containers that stabilize like urea or phosphoric acid.
Facts About Hydrogen Peroxide
Acidic Nature of H2O2
Hydrogen peroxide behaves like a weak dibasic acid. The pH scale value of H2O2 is lower than that of water. The second dissociation of hydrogen is very weak. It cannot be protonated in an aqueous solution.
Oxidizing Property of H2O2
Hydrogen peroxide is a strong oxidizing agent in both acidic and basic mediums. The redox reaction potentials of H2O2 are given below in the table.
| H2O2 + 2H+ + 2e → 2H2O | E0 = 1.77 volt |
| O2 + 2H+ + 2e → H2O2 | E0 = 0.68 volt |
| HO2− +H2O + 2e → 3OH− | E0 = 0.87 volt |
Due to the high positive potentials of hydrogen peroxides, it can easily oxidize iron (II) to iron (III), bromide to bromine, and iodide to iodine.
Reducing Property H2O2
In the presence of very strong oxidizing agents like chlorine, permanganate, or dichromate in an acid medium, H2O2 oxidizes to oxygen. Therefore, it shows a reduced property.
Uses of Hydrogen Peroxide
- Industrial peroxide is mostly used in medicine and is a bleaching agent in our everyday life. About 30% of peroxide is used as a bleaching agent for textiles, paper, pumps, lather, and oil industries.
- A large quantity of about 33% uses in the manufacture of borax, epoxides, propylene oxide, and other chemicals.
- In environmental science, peroxide is used in pollution control during the treatment of sewage cleanup and waste.
- It uses as a mild antiseptic to prevent the small cuts, scapes, and buns on the skin.
- Hydrogen peroxide is also used as a mouth rinse in medicine to the freshness or as a mouth cleaner.