Facts About Hydrogen Element
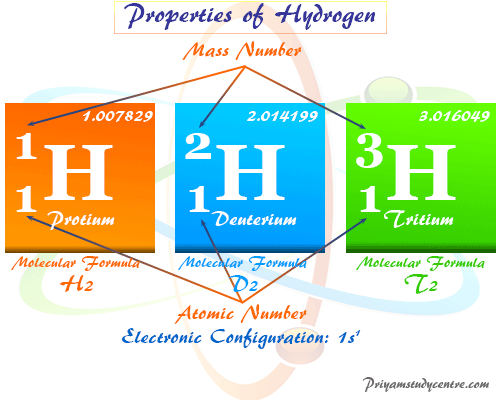
Hydrogen is the only chemical element in the periodic table in which the valence electron is under the direct influence of the nucleus or there is no shielding electron. The ns1 electronic configuration justifies the various facts, chemical properties, and the position of the hydrogen element in the periodic table. Elemental hydrogen has the chemical symbol ‘H‘ while molecular form or dihydrogen has the molecular formula H2. The colorless, odorless, flammable hydrogen gas molecule or liquid has been uses mainly for making fuel cells and various industrial production processes. Hydrogen has three natural isotopes having the chemical name protium (1H1), deuterium (1H2), and tritium (1H3). Under ordinary conditions, due to the presence of one valence electron in the 1s-orbital, it forms a wide number of common chemical bonding such as covalent, ionic, bridge bonds, and hydrogen bonding.
The atom of hydrogen element has a very simple structural model because it contains one electron and one proton with atomic number = 1 and atomic weight or mass = 1.007829. Hydrogen forms more chemical compounds than any other periodic table elements, including carbon. Therefore, it forms compounds of one kind or another with nearly every other active periodic table element.
Hydrogen Production
Hydrogen can be produced in the laboratory by the action of metals on water, acids, and bases. It is also obtained as a byproduct in the electrolytic manufacturing of caustic alkalies from brine.
The alkali metals and alkaline earth metals react with water in cold conditions evolving hydrogen while magnesium and aluminum give this product when react with boiling water.
2Na + 2H2O → 2NaOH + H2
Ca + 2H2O → Ca(OH)2 + H2
Al + 6H2O → 2Al(OH)3 + 6H2
Acids like dilute hydrochloric acid and dilute sulfuric acid react with certain active metals like zinc and iron to form salt and evolve H2 gas. Therefore, such acids or substances that contain these kinds of acids should not be kept in metal containers.
Metal + Dilute acid → Salt + Hydrogen gas
Zn + H2SO4 → ZnSO4 + H2
Strong bases like sodium hydroxide and potassium hydroxide react with active metals to produce hydrogen gas. Therefore, these bases should not be kept in metal containers made of active metals.
Metal + Base → Salt + Hydrogen gas
Zn + 2NaOH → Na2ZnO2 + H2
The hydrolysis of calcium hydride is also a convenient process for the production of hydrogen.
CaH2 + 2H2O → 2Ca(OH)2 + 2H2
Steam may be decomposed by coke at high temperatures to give water gas or a mixture of hydrogen and carbon monoxide.
C + H2O → CO + H2
Electrolyzer for Hydrogen Production
Very pure hydrogen and oxygen can be prepared by electrolysis of 20% caustic soda (sodium hydroxide) or caustic potash (potassium hydroxide) solution in a steel electrolyzer using iron electrodes. The anode of such an electrolyzer is nickel-plated to prevent oxidation. The anode and cathode are separated by porous diaphragms to prevent the diffusing and mixing of hydrogen with oxygen because the mixture is explosive.
Production from Natural Gas
A large amount of hydrogen is now produced from the reforming of hydrocarbons present in the natural gas by steam in the presence of a nickel catalyst at about 900 °C. Some methane gas and carbon dioxide are also formed during the reforming of natural gas.
CnH2n+2 + nH2O → nCO + H2
The mixture of the gases is cooled to 350 °C and entered into a shift converter. Most of the carbon monoxide is catalytically converted to carbon dioxide by steam. The gas is ultimately cooled and carbon dioxide can be removed by absorption.
CO + H2O → CO2 + H2
The last trace of CO can be removed by methylation or conversion of CO to methane by nickel catalyst. Therefore, the purity of hydrogen by such a production process is very high.
CO + 3H2 → CH4 + H2O
Where is Hydrogen Found?
In 1766 Henry Cavendish, an English chemist and physicist discovered the most combustible element or H2 molecule. The name hydrogen was given from the origin of Greek words meaning ‘water maker’.
Besides the common physical states of matter like solid, liquid, and gas in the Earth’s universe, H2 is a gas molecule at room temperature. It is the third most abundant chemical element after oxygen and silicon and 2nd most abundant gas molecule after oxygen. Therefore, the combined form is found widely in the earth’s crust and oceans.
Hydrogen in Periodic Table of Elements
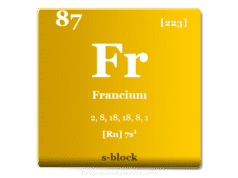
The ns1 electronic configuration justifies the position of hydrogen in period-1 and group-1 with the alkali metals family (lithium, sodium, potassium, rubidium, cesium, and francium). The ionization energy of hydrogen is also similar to that of alkali metals.
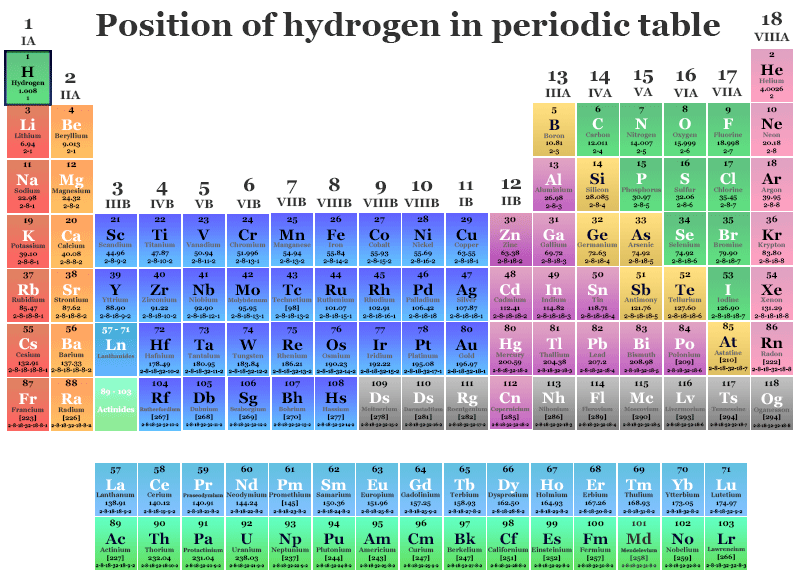
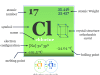
If we consider the 1s1 configuration of hydrogen, it is just one electron short of the next noble gas element helium. Therefore, it may be placed in group 17 with the halogen family such as fluorine, chlorine, bromine, and iodine. All these elements have very high electronegativity and electron affinity.
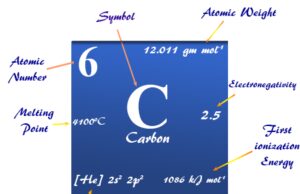
Due to the presence of the half-filled valence electrons, it is also placed in group 14 with the carbon family. Therefore, it forms a wide number of covalent molecules with a wide range of polarity.
Oxidation State of Hydrogen
The most common oxidation number of hydrogen element = +1, but due to unique characteristics, it also shows a −1 oxidation state.
For example, in lithium hydride (LiH), sodium hydride (NaH), cesium hydride (CsH), and calcium hydride (CaH2), the oxidation state or number of the hydrogen element = −1.
Isotopes of Hydrogen
Hydrogen has three isotopes having the chemical names protium, deuterium, and tritium. The chemical formulas of these isotopes are 1H1, 1H2, and 1H3 respectively. Terrestrial elemental form contain about 0.0156% of deuterium atoms. However, tritium occurs naturally in very low quantities but it is continuously formed in the upper atmosphere by nuclear reaction induced by cosmic rays.
These three isotopes form three covalent gas molecules like dihydrogen, dideuterium, and ditritium. These isotopes are used as an alternative fuel for engines or renewable energy sources for our environment.
Hydrogen Properties
At standard conditions, it forms a diatomic molecule called dihydrogen with the chemical formula H2. The colorless, odorless, tasteless, non-toxic, and highly combustible hydrogen has a 1s1 electronic configuration. The 1s1 electronic configuration justifies various facts and properties of hydrogen. It also justifies the position of such elements in the periodic table.
| Hydrogen |
|
| Symbol | H |
| Discovery | Henry Cavendish in 1766 |
| Name derived from | The Greek words hydro and genes, meaning water forming |
| Allotrope | H2 |
| Common isotope | 1H2 (deuterium), 1H3 (tritium) |
| Crystal structure | Hexagonal crystal lattice |
| Periodic properties | |
| Atomic number | 1 |
| Electron per shell | 1 |
| Atomic weight | 1.008 |
| Electronic configuration | 1s1 |
| Group | 1 |
| Period | 1 |
| Block | s-block |
| Physical properties | |
| State at 20 °C | Gas |
| Melting point | −259.16 °C, −434.49 °F, 13.99 K |
| Boiling Point | −252.88 °C, −423.18 °F, 20.27 K |
| Density | 0.000082 g cm−3 |
| Chemical properties | |
| Atomic radius (non-bonded) | 1.10 Å |
| Covalent radius | 0.32 Å |
| Oxidation number or states | 1, −1 |
| Ionization energy | 1st: 1312.05 kJ mol−1 |
| Electron affinity | 72.769 kJ mol−1 |
| Electronegativity | 2.19 (Pauling scale) |
| Molar heat capacity |
28.836 J mol-1 K-1 |
| CAS number | 133-74-0 |
Chemical Properties
The hydrogen molecule is a very stable species because very small quantities of atomic form are formed when heating hydrogen gas at about 2000 °K in 1 atm pressure. The most reactive atomic form may produced by passing H2 over heated platinum or palladium wire at 2000 °K or exposing a mixture of mercury and H2 in a high-energy mercury lamp.
Such a form of the element is used in the high-temperature welding of metals because during recombination of hydrogen atoms releases a large amount of heat.
Among various ionized forms, the proton H+ and hydride ion H− are common. The formation of a proton from the H atom required very high ionization energy. Chemically the H+ is not a stable species because the size of the H+ ion is very small. However, it can stabilize when bonded with water molecules.
The addition of an electron to the 1s orbital of hydrogen produces the hydride ion. However, the formation of H− is much less favorable due to low electron affinity and very high bond dissociation energy. The gain of electrons to form hydride ions may occur in the hydride of alkali and alkaline earth metals.
Important Facts About Hydrogen
Molecular H2 is a very stable and nonreactive chemical species due to high bond energy but the atomic form is the most reactive species. Only one percent of atomic forms are present in the H2 gas molecule.
The half-life of the atomic form is nearly 1 second at 0.2 mm pressure. A huge specific heat (432.6 kJ mol−1) is used for the recombination of atomic H. This process is used for high-temperature welding of tantalum and tungsten metals.
Due to high reactivity, it functions as a strong reducing agent. It reduces different types of common chemical compounds like ethylene, acetylene, hydrogen peroxide, and metal ions.
Hydrogen Element in the Atmosphere
Hydrogen is a unique type of chemical element that is not found free in the earth’s atmosphere but is widely used in everyday life. The kinetic energy of H2 molecules at the earth’s temperature is sufficient to escape from the earth’s gravitational energy. Therefore, the density of H2 decreases in our environment.
Hydrogen Spectrum
Hydrogen atoms absorb energy to shift their valence electrons particles to different energy levels. The electrons in higher energy levels are relatively unstable and hence drop back to the lower energy level to produce the electromagnetic spectrum or H-spectrum.
Nuclear Spin Isomers
When the nucleus of an atom contains an odd number of nucleons, the nucleus has a resultant spin. If such two atoms combine to form a diatomic molecule, the nuclei may have their spin parallel or anti-parallel. It is called nuclear spin isomerism.
- The molecule in which the two nuclei have a parallel spin is called an ortho-isomer.
- The molecule with antiparallel nuclear spins is called a para-isomer.
Such nuclear spin isomer is found in H2, D2, T2, N2, O2, etc. The conversion of para-H2 to the ortho-H2 compound is normally very slow. They involved the forbidden transition between two energy states of the different spin municipalities.
Uses of Hydrogen Gas
Elemental hydrogen and its compounds are widely used in the different types of fuel cells and industrial production processes. Hydrogen isotopes are also used widely in chemical and nuclear industries. The most common uses of elemental and molecular hydrogen and its isotopes are given below,
Uses in Industry
- Gaseous H2 is used for the manufacture of ammonia.
- Gaseous H2 is also an important chemical compound used for the manufacturing of hydrochloric acid, sulfuric acid, nitric acid, methyl alcohol, etc.
- Atomic hydrogen torches are used for welding of high melting metals like tantalum and tungsten.
- H2 gas is also used for the reduction of metal oxides. A direct reduction of iron ore by H2 is used for the manufacturing of iron.
- The liquid H2 molecule is used in the propulsion of rockets.
- In bubble chambers, liquid hydrogen is used to study high-energy particles.
- Hydrogen oxygen type mixture is used in the fuel cells for the production of renewable energy which uses widely electric vehicles.
Laboratory Uses
- In a hydroformylation reaction, H2 may add a formyl group into alkenes. The produced formyl compound subsequently changed to form different types of alcohol. These alcohols are used for the preparation of PVC resin and detergents.
- Methyl alcohol prepared from H2 gas is subsequently oxidized by the oxo process to form formaldehyde which is a starting material for the synthesis of plastics.
- Hydrogen electrodes are used for the determination of the pH of an unknown solution.
- The hydrogen spectrum is also used to analyze different types of physical or chemical properties of hydrogen-containing chemical compounds.
Uses of Liquid Hydrogen
Hydrogen play an important role in the near future by providing an alternative renewable resource of energy. Therefore, when nonrenewable fossil fuel reserves are exhausted, liquid hydrogen provides the most promising alternative source of energy. It is also used as a medium for the transport and storage of energy.
Using it as a fuel provides great advantages because it is free from pollution. The only problem lies with the storage and transmission of bulk quantities of liquid hydrogen. However, vacuum-insulted cryogenic transmission tanks have been successfully used to store huge quantities of liquid H2 for Us space programs. Pipelines and road transmission tankers are also designed for transportation.
Fuel cells using liquid H2 for the regeneration of electrical energy have been operated on a commercial basis to fulfill the demand for electricity. Non-electrolyte methods of producing cheap hydrogen from water are also under extensive search and research activity.
Uses of Deuterium
- Deuterium is a common isotope of chemical element hydrogen which is used mainly for the production of heavy water.
- The heavy water used as a moderator and coolant in nuclear power reactors is obtained commonly from deuterium molecules.
- Deuterium oxide or heavy water is the main source of deuterium compounds.
- To study the reaction mechanisms, we also widely use deuterium and its compounds.
Uses of Tritium
Tritium is made artificially by bombarding 3Li6 with neutron particles.
3Li6 + 1n0 → 2He4 + 1T3
Tritium is β-active and cannot be stored for a long time. Therefore, it can be used mainly for research purposes. The most common uses of tritium are listed below:
- Tritium is widely used as a tracer element due to its radioactivity.
- Tritium extensively uses hydrological studies on the moment of groundwater in the earth’s environment
- It is also used to study the absorption of crystalline metals and multiphase alloys in autoradiography.
- The isotope tritium is used to study the reaction mechanism, reaction kinetics, and homogeneous catalysis.
Frequently Asked Questions
What is Hydrogen?
Hydrogen (symbol H) is the only chemical element in the periodic table in which the valence electron is under the direct influence of the nucleus or there is no shielding electron. Therefore, the ns1 electronic configuration justifies the various facts, chemical properties, and the position of this element in the periodic table.
What is the symbol of hydrogen?
Elemental hydrogen has the chemical symbol ‘H’ while molecular form has the chemical formula H2.
How is hydrogen made from natural gas?
A large amount of hydrogen can be made from the reforming of hydrocarbons present in the natural gas by steam in the presence of a nickel catalyst at about 900 °C.
CnH2n+2 + nH2O → nCO + H2
Some methane molecules and carbon dioxide are also formed during the reforming of natural gas.
CO + H2O → CO2 + H2
CO + 3H2 → CH4 + H2O
Where is hydrogen in the periodic table?
The ns1 electronic configuration justifies the position in period-1 and group-1 with the alkali metals family (lithium, sodium, potassium, rubidium, cesium, and francium). The similar ionization energy also justifies the position of hydrogen with alkali metals.
Who discovered the hydrogen element?
The most combustible element or H2 molecule was discovered in 1766 by Henry Cavendish, an English chemist and physicist. The name hydrogen was given from the origin of Greek words meaning ‘water maker’.
What is Hydrogen Used for?
Elemental and green hydrogen are widely used in the different types of fuel cells and industrial production processes. In the chemical industry, H2 gas is used for the production of ammonia, hydrochloric acid, sulfuric acid, nitric acid, methyl alcohol, etc. It is a good source of renewable fuel that is used commonly the propulsion of rockets and the production of fuel cells.