Nitric Acid (HNO3)
Nitric acid (chemical formula HNO3) is colorless, fuming, highly corrosive liquid that uses mostly for the production of fertilizers. Concentrated nitric acid is the most important heavy chemical used in the fertilizer production plant and explosives manufacturing process. Pure concentrated nitric acid is a high density toxic solution that burns our skin. The pH scale value of HNO3 is very low. Nitric acid is a common laboratory reagent that stores and uses millions of tonnes each year. The freezing point of HNO3 is −41.6 °C and the boiling point of 82.6 °C. The concentrated water solution of nitric acid has properties of photochemical decomposition. It turns brown by absorbing liberating nitrogen dioxide (NO2). In learning chemistry, nitric acid (HNO3) in the gas phase contains a planner structure but the nitrate ion has a planner symmetrical structure with a negative charge on resonance hybrid.
Where to Find Nitric Acid?
Nitric acid is manufactured during the catalytic oxidation of ammonia (NH3) by the Oswald process or distillation of sodium or potassium nitrate with concentrated sulfuric acid. In the chemical laboratory, it is the most commonly used acid after hydrochloric acid and sulfuric acid. Like other acids, it is also a pollutant of our environment that causes acid rain or snow.
Commercially, 98 percent concentrated nitric acid is converted to yellow or red fuming acid by dissolving extra nitrogen dioxide in 98 percent acid solutions.
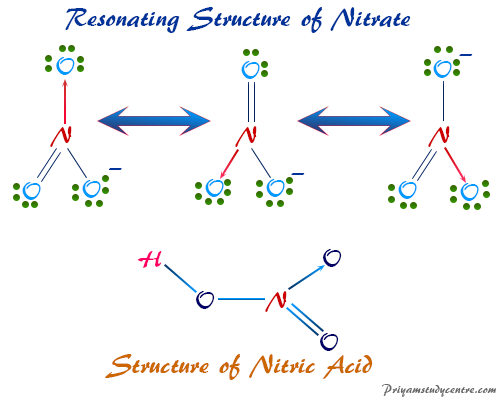
Nitric Acid Lewis Structure
Nitric acid (HNO3) in the gas phase contains a planner structural formula but the nitrate ion has a planner symmetrical structure with a negative charge on resonance hybrid. The same general structure holds probably in solid forms of HNO3.
The first two N-O chemical bond distances in HNO3 are equal but the third N-O polar bond distance is longer than the first two due to low bond energy corresponding to the single bond.
Structure of Nitrite Ion
The resonance hybrid of nitrate ion consists of a roughly sp2 hybridized orbital at which the nitrogen atom involves resonance hybrids with a symmetrical planner structure. The N-O bond distance in HNO3 1.22, consists of a bond order of 1 and 1/3.
Laboratory Preparation of HNO3
In the laboratory, nitrogen dioxide can be produced by the thermal decomposition of copper(II) nitrate. It can be passed through water or hydrogen peroxide to produce nitric acid.
2 Cu(NO3)2 → 2 CuO + 4 NO2 + O2
2 NO2 + H2O → HNO2 + HNO3
Alternatively, 50 g of potassium nitrate (KNO3) and 25 ml of concentrated sulphuric acid are taken in a round bottom flask in the laboratory. It can be heated to about 200 °C to produce HNO3.
KNO3 + H2SO4 → HNO3 + KHSO4
Nitric Acid Production
Industrially, the production of pure nitric acid (molar mass or molecular weight = 63 g/mol) happens by heated ammonia with a suitable catalyst (Ostwald process). Ammonia undergoes different types of oxidation reactions under various conditions. For example, in the air, ammonia reacts but little to form nitrogen. In pure oxygen, it burns to form nitrogen and water.
The production of pure HNO3 by the Ostwald process is carried out by following steps:
When a mixture of ammonia and air (1:7) is passed over a platinum gauze (10% rhodium) chemical catalyst heated at 750°C, ammonia can be oxidized by oxygen in air to produce nitric oxide. The liberated specific heat keeps the catalyst hot.
4 NH3 + 5 O2 → 4 NO + 6 H2O
The heated nitric oxide cooled and is further oxidized by atmospheric oxygen to form nitrogen dioxide.
2 NO + O2 → 2 NO2
Nitrogen dioxide passed into warm water or soluble under pressure to yield a 60 percent dissolved solution of nitric acid and nitric oxide.
3 NO2 + H2O → 2 HNO3 + NO
Commercially available concentrated HNO3 is 98 percent concentrated. Fuming HNO3 contains additionally dissolved NO2 with a yellow colour.
Properties of Nitric Acid
Pure HNO3 is a colourless liquid. The pure or concentrated aqueous solutions of nitric acid have the properties of photochemical decomposition with a very high dielectric constant, a good ionizing solvent for electrolytes. It is also a powerful oxidizing agent for oxidation.
| Properties of nitric acid (HNO3) | |
| Chemical formula | HNO3 |
| Molar mass | 63.012 g mol−1 |
| Appearance | colorless, yellow, or red fuming state |
| Melting point | −42 °C or −44 °F or 231 K |
| Boiling point | 83 °C or 181 °F or 356 K) |
| Density | 1.51 g cm−3 |
| Conjugate base pair | nitrate ion (NO3−) |
| Acidity (pKa) | 1.4 |
Chemical Properties
A dilute aqueous solution of HNO3 (molarity below 2M) behaves like a strong acid with little oxidizing power. Dissociation is nearly 93 percent at 0.1M concentration. Therefore, only magnesium and manganese liberate hydrogen from the dilute solution of HNO3.
If the concentration is greater than 2M, HNO3 is a powerful oxidizing agent. Dilute HNO3 attracts most metals except gold, platinum, iridium, rhodium, and rhenium to form salt and evolved hydrogen gas. However, chromium, aluminum, iron, and some extent of copper are rendered for liberating hydrogen due to the formation of the oxide film.
Metal + Diluted HNO3 → nitrate salt + hydrogen
For example, when it reacts with magnesium, manganese, and zinc, it produces respective nitrate salts and hydrogen.
Mg + 2 HNO3 → Mg(NO3)2 + H2
Mn + 2 HNO3 → Mn(NO3)2 + H2
Zn + 2 HNO3 → Zn(NO3)2 + H2
Concentrated HNO3 in the presence of sulfuric acid attacks aromatic hydrocarbon and their derivatives to form nitro compounds.
Aqua Regia
The mixture of concentrated HNO3 (3 volumes) and concentrated HCl (1 volume) is known as aqua regia. Aqua regia is a powerful oxidant due to the presence of free chlorine and NOCl
Aqua regia can dissolve gold or platinum metals through the action of nascent chlorine and ClNO. Therefore, such solutions are used for refining gold or platinum metals and cleaning glassware in laboratories.
Uses of Nitric Acid
- The main storage consumption is about 80 percent of strong nitric acid used in the manufacture of ammonium nitrate (NH4NO3) which is generally used as an acidic fertilizer for plants, and explosive in mining.
- Nitric acid is also used (5 -10 percent) in making cyclohexanone, and caprolactam as a route to nylon.
- It is confined to nitration in chemical reactions for the production of nitroglycerine, nitrocellulose, and trinitrotoluene (TNT).
- It is also used in the pickling of metal surfaces, as an oxidizer in rocket fuel, and in the manufacture of various nitrate salts.
- The formation of red-fuming nitric acid molecules is found by dissolving N2O4. It is a superior nitrating laboratory reagent in chemistry.
- A mixture of concentrated HNO3 and H2SO4 is also used to make a strong nitrating chemical compound, name nitronium ion (NO2+).