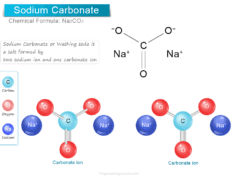
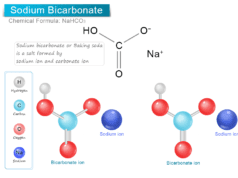
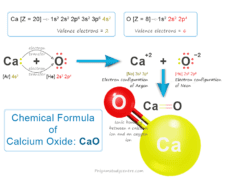
Sulfuric Acid Chemical Formula
Sulfuric acid, also called sulphuric acid or hydrogen sulfate (chemical formula H2SO4) is a commercially important dense, colorless, oily, corrosive liquid and a strong dibasic acid that is used largely in chemical plants for fertilizer production or manufacturing. It is also called the oil of vitriol because it is prepared by roasting green vitriols or iron (II) sulfate. Commercially, sulfuric acid can be manufactured by the contact process. Oleum or fuming H2SO4 is obtained by dissolving sulfur trioxide (SO3) gases or vapors in a concentrated H2SO4 solution. In chemistry laboratories, it is always diluted by slowly pouring water with stirring. Concentrated 98 percent of sulfuric acid uses as an effective dehydrating or dissociation reagent with strong oxidizing properties.

Annual about 150 million tons of production of sulfuric acid from different sources in the whole world. It leaves 250000 tons of sulfur per year in our environment. Therefore, it is responsible for producing pollutants like acid rain or snow in the earth’s atmosphere.
Sulfuric Acid Lewis Structure
The Lewis structure of sulfate ion and sulfuric acid (H2SO4) contains tetrahedral with sp3 hybridized sulfur atom. The electronic structure of the sulfate ion and acid are given below picture,
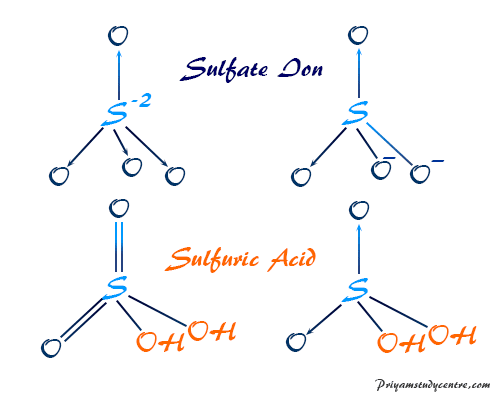
- In structure A, sulfur has the 6+2 = 8 valence shell electrons which are distributed in four sp3 orbitals. This orbital makes four coordinate chemical bonding with four oxygen atoms.
- In structure B, sulfur has the 6 + 1 = 7 valence shell electrons. Among these two electron particles, pairs used in two sp3 orbitals make the coordinate bonds. Two other hybrid quantum orbitals with one electron each overlap with oxygen p-orbitals to make one sigma bond each.
Chemical Properties of H2SO4
Anhydrous sulfuric or pure sulphuric acid is a colorless, heavy, viscous liquid with a high dielectric constant. It has a very weak pH level and neutralized with strong bases like sodium hydroxide or potassium hydroxide.
| Physical and chemical properties | |
| Molecular formula | H2SO4 |
| Molar mass | 98 gm/mol |
| Melting point | 10.4 °C |
| boiling point | ∼ 300°C |
| Density | 1.83 gm/ml |
| Viscosity | 24.5 centipoise |
| Solubility in water | soluble or miscible |
| Conjugate base | bisulfate |
The molecules are bound by strong hydrogen bonding in both the liquid. Solid form producing hydrogen ions or protons in a water solution with several ionization constants presented as,
- H2SO4 + H2SO4 → H3SO4+ + HSO4−
K (10 °C) = 1.7 × 10−4 mol2 kg−2 - H2SO4 + H2SO4 → H3O+ + HS2O7−
K (10 °C) = 3.5 × 10−5 mol2 kg−2 - H2S2O7 + H2SO4 → H3SO4+ + HS2O7−
K (10 °C) = 7 × 10−2 mol2 kg−2
Hydration of concentrated H2SO4 liberates a large amount of specific heat.
In learning chemistry, due to the low pH scale of sulfuric acid, it always dilutes by slowly treatment with water by stirring but reverse addition may cause explosive of the acid mixture by the stream formation. At low temperatures, this forms of H2SO4 several crystalline solid hydrates.
Chemical Reaction
- Concentrated H2SO4 has a great affinity for water and forms several crystalline hydrates.
- It serves as an excellent drying reagent. Cellulose materials such as paper, cotton, wood, and sugar (C12H22O11) are dehydrated by the H2SO4 solution.
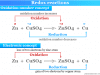
- Metals above hydrogen atom in the electrochemical series or metals with positive standard redox potential do not dissolve in cold or dilute sulfuric acid.
- The chemical elements like gold and silver in the periodic table dissolve in hot conditions because hot acid works as a good oxidant.
Sulfuric Acid Production Process
The contact process or chamber process is used for the manufacture of sulfuric acid based on the oxidation of SO2 to SO3 with the vanadium pentoxide chemical catalyst promoted by potassium sulfate,
SO2 + ½O2 → SO3 ΔH0 = − 98 kJ mol−1.
Due to the exothermic reaction, the contact process gives higher equilibrium conversation at low temperatures and high pressure. But at low temperatures below 400°C, the rate of reaction becomes very slow and the V2O5 catalyst is inactive.
The conversation was affected in four stages and worked best around 450 °C to 550 °C.
The sulfur dioxide produced in the contact process is absorbed by 98 percent concentrated H2SO4. The fuming nature of the substance or oleum is produced by dissolving sulfur trioxide in a concentrated sulfuric acid solution.
Uses of Sulfuric Acid
- like nitric acid, sulfuric or sulphuric acid industrially manufactured by contact process has also great industrial importance in fertilizer production. Sulfuric acid is widely used in the fertilizer industry to make ammonium sulfate.
- It uses for the production of supersulfate which uses to make many inorganic and organic compounds like alcohol, ester, ethers, sulfates, and other acids.
- It is used widely in cleaner materials for home, in car batteries, etc.
- Fuming sulfuric acid uses for sulfonation in the organic synthesis equation that introduces −SO3H group in benzene or derivative of benzene compounds.
- Huge quantities of sulfuric acid are used in the world in many chemical plants for the manufacture of paints, pigments, dyestuffs, drugs, fibers, detergents, explosives, lead-acid storage batteries, petroleum refining, and metallurgical processes in chemistry.