Acetic Acid Solution
Acetic acid also called ethanoic acid has the chemical formula CH3COOH and is prepared industrially by various methods. It is the second simplest carboxylic acid after formic acid. Acetic acid solution is miscible in all proportions with water, ethanol, and ether. The solubility of CH3COOH arises due to the formation of hydrogen bonding with the water molecule. Commonly, acetic acid solution is used as a solvent for the preparation of organic compounds like acetate, acetone, acetic anhydride, acetaldehyde, alcohol, vinegar, etc. Glacial acetic acid is a pungent corrosive liquid solution having a molar mass of 60.052 g mol−1. Ethanoic acid is mildly corrosive to metals (iron, magnesium, and zinc) and forms metal acetate and hydrogen gas. But when reacts with aluminum, it forms a passivating acid-resistant film of aluminum oxide. Therefore, aluminum tanks can be used for transporting such acid solutions.
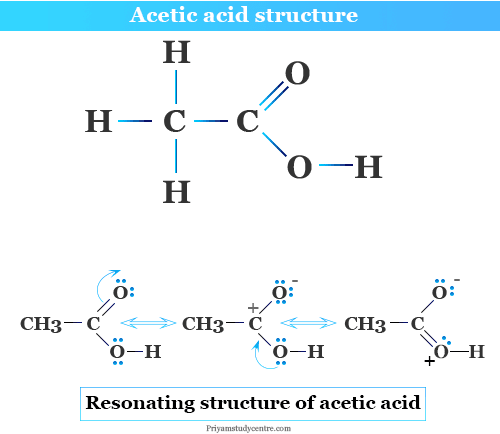
Structure of Acetic Acid
Acetic acid is the second simplest carboxylic acid after formic acid which contains one methyl group and carboxylic acid group in its structure. These two functional groups are connected by single covalent chemical bonding in the CH3COOH molecule.
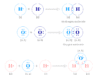
The lower members of monocarboxylic acids are far less volatile than is to be expected from their molecular. They form an eight-membered ring or cyclic dimer by intramolecular hydrogen bonding.
- In solid-state, the CH3COOH molecules can interconnected by intermolecular hydrogen bonding.
- In vapour phase and aqueous solution, CH3COOH molecules can interconnected by intramolecular hydrogen bonding to form an eight-membered ring or cyclic dimer.
Properties of Acetic Acid
Acetic acid ionizes or donates its proton to water to form the conjugate base like acetate (CH3COO−) ion that shows two equivalent resonating structures.
CH3COOH → CH3COO− + H+
Due to the release of the proton (H+), it shows a mild acidic character. Therefore, it is a weak monoprotic acid.
| Acetic acid |
||
| IUPAC name | Ethanoic acid | |
| Chemical formula | CH3COOH | |
| CAS Number | 64-19-7 | |
| Molar mass | 60.052 g mol−1 | |
| Appearance | Colourless liquid | |
| Density (g/cm3) | Liquid | Solid |
| 1.05 | 1.27 | |
| Melting point | 16 to 17 °C | |
| Boiling point | 118 to 119 °C | |
| Solubility | Miscible in water, ethanol solution | |
| Acidity (pKa) | 4.756 | |
| Conjugate base | Acetate (CH3COO−) | |
| Viscosity | 1.22 mPa s | |
| Dipole moment | 1.74 D | |
| Heat capacity | 123.1 J K−1 mol−1 | |
| Entropy | 158.0 J K−1 mol−1 | |
The internal energy of the anion is lower than the unionized acid. In the case of alcohol, there is no resonance hybrid form in the alkoxide ion. Therefore, it is a stronger acid than alcohol. Therefore, the standard free energy for the ionization of monocarboxylic acid will be more negative than that of alcohol.
Solubility of Acetic Acid
Liquid acetic acid is a polar protic solvent but is miscible in polar and non-polar solvents such as water, chloroform, and hexane. Therefore, it can be used as a good solvent in many chemical industries.
The first four monocarboxylic acids are very soluble in aqueous medium. The solubility in water decreases as the molecular weight of carboxylic acids increses. The solubility of CH3COOH can be explained by the formation of hydrogen bonding with water molecules.
Acetic Acid Uses
- Acetic acid is mostly used as a solvent for preparing acetates, acetone, and acetic anhydride.
- It is stable toward oxidizing agents and a useful solvent for the chromium trioxide oxidation process.
- Acetylene passes over worm ethanoic acid in the presence of mercuric ions as a chemical catalyst to form vinyl acetate and ethylidene diacetate. Vinyl acetate is used mostly in the plastic industry for the production of plastic toys and daily used equipment.
- Vinegar (6 to 10 percent aqueous solution of ethanoic acid) is commonly used in food preparation, in particular pickling liquids, vinaigrettes, and other salad dressings.
Synthesis of CH3COOH
One of the earliest methods for preparing glacial acetic acid was by destructive distillation of wood to find pyroligneous acid. This contains 10% of acid solution treated by neutralizing with lime and distilling as the volatile compounds.
This volatile compound is a mixture of methanol and acetone. The distillation of methanol and acetone with sulfuric acid gives dilutes CH3COOH.
Industrially, CH3COOH is prepared from several organic compounds like alcohol, acetaldehyde, and n-butanol. Some synthesis methods are given below the picture,
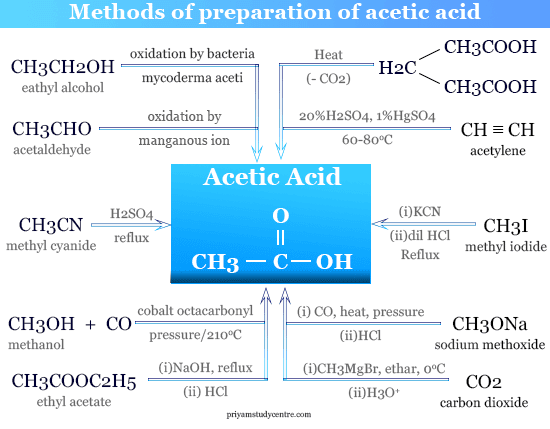
Acetaldehyde to Acetic Acid
CH3COOH is prepared by air oxidation of acetaldehyde in the presence of a manganous ion.
CH3CHO + [O] → CH3COOH
Oxidation of n-Butane
Acetic acid can be prepared by air oxidation of liquid hydrocarbon (n-butane) under pressure and at 130-230 ºC temperature in the presence of a suitable chemical catalyst like manganous ion.
Carbonylation of Methanol
Acetic acid can be synthesized industrially during the reaction between carbon monoxide and methanol under pressure in the presence of cobalt octacarbonyl at about 210 °C.
CO + CH3OH → CH3COOH
Carbonylation of methanol can also be carried out in three steps. A metal carbonyl catalyst such as cobalt octacarbonyl is needed in step 2.
- Step I: CH3OH + HI → CH3I + H2O
- Step 2: CH3I + CO → CH3COI
- Step 3: CH3COI + H2O → CH3COOH + HI
Oxidation of Ethylene
Ethylene can be oxidized to form acetic acid catalyzed by a palladium catalyst on a heteropoly acid such as silicotungstic acid.
CH2=CH2 + O2 → CH3COOH
Anaerobic Fermentation
Some anaerobic bacteria can also convert sugars into acetic acid without creating intermediate alcohol ethanol.
C6H12O6 → 3 CH3COOH
How to Make Vinegar from Acetic Acid?
Vinegar is the 6−10 percent of the aqueous solution of acetic acid used to preserve food. Vinegar also contains a small amount of flavoring compounds. It can be made in several ways.
Vinegar solution in organic chemistry is generally prepared by the oxidation of wort or ethyl alcohol by means of the bacteria Mycoderma aceti.
CH3CH2OH + O2 → CH3COOH + H2O
Quick Vinegar Process
- Beech shavings in barrels can be moistened with strong vinegar in the presence of bacteria.
- A 10 percent aqueous solution of ethanol, phosphates, and inorganic salts which is necessary for the fermentation then poured into the barrel.
- Ethanol is thereby oxidized to form acetic acid. A plentiful supply of air or oxygen is necessary otherwise the oxidation is incomplete and acetaldehyde is produced.
Chemical Reactions of Acetic Acid
Acetic acid can show many chemical reactions with various inorganic and organic reagents. When heating above 440 °C, ethanoic acid can be decomposed to form carbon dioxide and methane or ketene and water.
CH3COOH → CH4 (methane) + CO2
CH3COOH → CH2=C=O (ketene) + H2O
Reaction with Metals
When acetic acid reacts with strong electropositive metals (lithium, sodium, magnesium, or zinc), it liberates hydrogen and forms acidic salt.
2 CH3COOH + 2 Na → 2 CH3COO−Na+ + H2
Reaction with Alcohols
Acetic acid can react with alcohols (methanol, ethanol, propanol, and butanol) to form esters. For example, it can react with ethanol to form ethyl acetate and water.
CH3COOH + CH3CH2OH → CH3CO2CH3 + H2O
All esters obtained from such monocarboxylic acid are mostly used as solvents for inks, paints, and coatings.
Oxidation of Ethanoic Acid
Etanoic acid is extremely resistant to oxidation but heating of CH3COOH by a specific heat with suitable oxidizing agents ultimately produces carbon dioxide and water.
CH3COOH → 2 CO2 + 2 H2O
Reduction Reactions
The reduction product of monocarboxylic acid depends on the nature of the reducing agents used in the reaction. Therefore, the heating of ethanoic acid in the presence of hydrogen iodide and red phosphorus under pressure produces ethane (C2H6).
A similar product is also obtained when acetic acid is heated with hydrogen under pressure at elevated temperatures in the presence of a nickel catalyst.
CH3COOH + 3 H2 → CH3−CH3 + 2 H2O
Other Reactions
Phosphorus trichloride, pentachloride, or thionol chloride can react with ethanoic acid to form acetyl chloride (CH3COCl).
3 CH3COOH + PCl3 → 3 CH3COCl + H3PO3
CH3COOH + PCl5 → CH3COCl + HCl + POCl3
CH3COOH + SOCl2 → CH3COCl + HCl + SO2
The electrolysis of a concentrated solution of sodium or potassium acetate can give ethane.
2 CH3COOK + 2 H2O → CH3CH3 + 2 CO2 + 2 KOH + H2