Alkenes in Organic Chemistry
Alkenes or olefins in organic chemistry are unsaturated hydrocarbon or organic compounds that contain at least one double bond along with single bonds in their molecule. From the definition, the double bond in alkenes is formed by sharing two pairs of electrons between two successive carbon atoms with the general molecular formula CnH2n. Industrially, alkenes can be prepared by creaking of hydrocarbons. The preparation of alkenes can also carried out by dehydration of alcohol, pyrolysis of ester, and Wittig reaction of carbonyl compounds. The double bond in alkenes is known as the olefinic or ethylenic chemical bond. Therefore, they are also called olefin. The olefin name of alkenes generally comes from ethylene because ethylene is called olefiant gas or oil-forming gas. It is formed oily liquids when treated with chlorine or bromine.
Generally, alkenes or olefins are colorless non-polar organic compounds but more reactive than alkanes. According to the IUPAC system of nomenclature, the class suffix of olefins is −ene. Therefore, the series becomes the alkene series. The cyclic form of alkene is called cycloalkene or cycloolefin.
Isomerism in Alkenes
Alkenes or olefins that contain four or more carbon atoms can form various structural isomers. These alkenes are also isomers of cycloalkanes or cycloolefins. The isomers of the most common alkene that contain one double bond are:
- Ethylene (C2H4): One isomer
- Propylene (C3H6): propylene (one isomer)
- Butene (C4H8): 3 isomers (1-butene, 2-butene, and isobutylene)
- Pentene (C5H10): 5 isomers (1-pentene, 2-pentene, 2-methyl-1-butene, 3-methyl-1-butene, 2-methyl-2-butene)
Many alkenes or olefins molecules also show cis-trans isomerism. For example, butene has two isomers cis-butene and trans-butene.
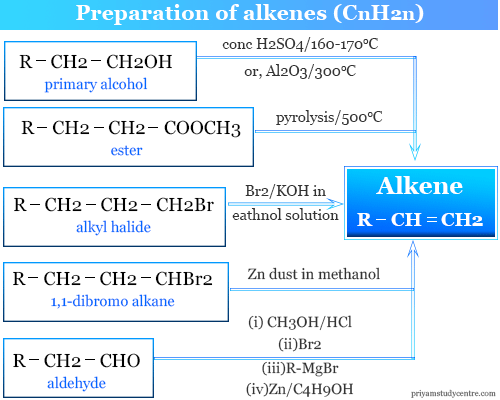
Preparation of Alkenes
Several alkenes like ethylene, propylene, and butylene can be prepared by the creaking of petroleum.
- For the industrial production of lower alkenes, the most suitable starting material is gas oil.
- During the production of higher alkenes, we used paraffin wax.
In the laboratory, alkenes can be prepared from different organic substances like alkanes, alcohol, esters, aldehydes, etc. In organic chemistry, alkenes can be prepared in the laboratory in the following ways,
- Dehydration of alcohol
- Pyrolysis of ester
- Cope reaction
- Alkyl halide to alkene
- From Grignard reagent
- Wittig reaction
Dehydration of Alcohol
Dehydration of alcohol is an important method in organic chemistry for the preparation of alkenes. The primary alcohol is heated with concentrated sulfuric acid at 170 °C to 180 °C to produce alkenes.
For example, ethanol can be heated with concentrated sulfuric acid at 170 °C to 180 °C to produce ethylene.
C2H5OH (ethanol) + H+ → C2H5OH2+ → H2O + C2H5+
C2H5+ (ethyl cation) → C2H4 (ethylene) + H+
Dehydration of secondary and tertiary alcohol is best carried out in the laboratory by using dilute sulfuric acid. Tertiary alcohol can polymerize under the influence of the concentrated sulfuric acid solution.
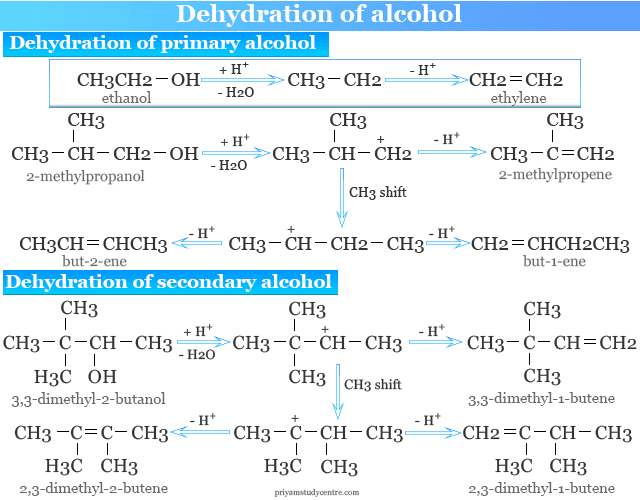
Dehydration of primary alcohol with an acid catalyst normally gives one alkene. However, secondary and tertiary alcohol gives a mixture of alkenes due to the rearrangement of the carbocation.
The rearrangement is generally avoided by dehydration of alcohol over alumina (Al2O3) in pyridine at 350 °C.
CH3CH2OH (ethanol) → H2C=CH2 (ethylene) + H2O
Saytzeff Rule
Rearrangement in the preparation of alkene often occurs with acid-catalyzed dehydration. Hence all three types of alcohol may behave in this way via a carbonium ion that may undergo methyl or hydride ion 1,2 shift.
The major product is in accordance with Saytzeff’s rule which is stated in two ways,
- The predominant product is the most substituted alkene which is the one carrying the largest number of alkyl substituents.
- Hydrogen is eliminated preferentially from the carbon atom which is joined to the least number of the hydrogen atom.
Pyrolysis of Ester
Most eliminations occur by polar mechanisms. The cyclic eliminations are uni-molecular non-polar chemical kinetics that take place in one step.
CH3−CH2−COOCH3 → CH2=CH2 + CH3COOH
When the compound is subjected to pyrolysis, the reaction proceeds via a cyclic transition state. This organic mechanism is supported by the fact that these chemical equilibrium reactions show a negative entropy of activation.
Cope Reaction
Cope reaction in organic chemistry is used for the preparation of alkenes by heating amine oxide at about 150 °C. The reaction was also carried out in the presence of dimethyl sulfoxide or tetrahydrofuran at room temperature.
Alkyl Halide to Alkene
Alkene like propene can be synthesized during the chemical reaction of propyl bromide with ethanolic potassium hydroxide.
CH3CH2CH2Br + KOH → CH3CH=CH2
Dehalogenation of 1,1 – di-halogen derivatives of alkanes using zinc dust and methanol produces alkenes.
CH3CH2CHBr2 → CH3CH=CH2
Zinc dust and methanol are also dehalogenations of 1,2- di halogen derivatives of alkanes used for the preparation of alkenes. Therefore, propene is prepared from propylene dibromide.
CH3CHBrCH2Br + Zn → CH3CH=CH2
From Grignard Reagent
Boord has prepared alkenes by conversion of an aldehyde into its chloro-ether.
R1CH2CHO + C2H5OH + HCl → R1CH2CHClOC2H5
This chloro-ether reacts with bromine followed by the Grignard reagent.
R1CH2CHClOC2H5 + Br2 → R1CHBrCHBrOC2H5
R1CHBrCHBrOC2H5 + R2MgBr → R1CHBrCHR2OC2H5
Finally, the product treated with zinc in n-butanol produced alkenes.
R1CHBrCHR2OC2H5 → R1CH=CHR2
This method is very useful for the preparation of alkene with a definite structure. An interesting point about it is the replacement of the ∝-chlorine atom by bromine when the ɑ-chloro ether bromination is in the β-position.
Wittig Reaction
How to Prepare Wittig Reagent?
The Wittig reagent, alkylidene-triphenyl phosphorane, is prepared by treating triarylphosphine with an alkyl halide in an ether solution. The resulting phosphonium salt is treated with a strong base such as C6H5Li, BuLi, NaNH2, NaH, and C2H5ONa.
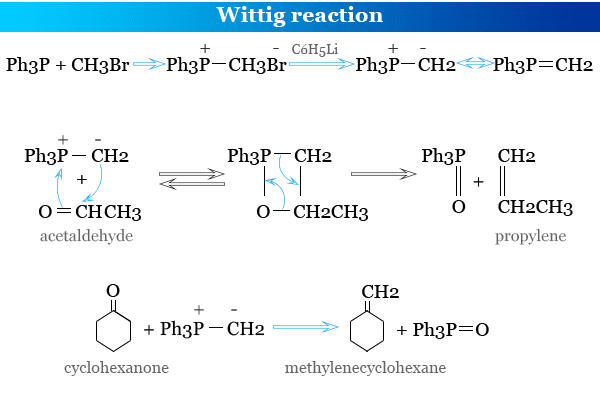
Preparation of Alkenes by Wittig Reaction
Wittig reagent is an important and useful chemical compound for the synthesis or preparation of alkenes during the reaction of aldehyde or ketones with triphenylphosphonium ylide or phosphorane molecule.
Ph3P=CHR1 + R2CHO → Ph3P=O + R1CH=CHR2
Frequently Asked Questions (FAQs)
How do you write an alkene formula?
The general molecular formula of an alkene = CnH2n
Where n = number of carbon atoms in one molecule of alkene.
Therefore, if an alkene has two carbon atoms or n = 2, its molecular formula
= C2H2×2
= C2H4 (ethylene)
When an alkene has three carbon atoms or n = 3, its molecular formula
= C3H2×3
= C3H6 (propylene)
Why are alkenes and alkynes more reactive?
In organic chemistry, unsaturated hydrocarbons (alkenes and alkynes) are more reactive than saturated hydrocarbons (alkanes) due to the presence of pi electrons or mobile electrons. Unsaturated hydrocarbon molecules can participate in a variety of addition reactions. They can also be used in polymer formation or participate in polymerization reactions.
What is Wittig reaction?
Wittig reaction in organic chemistry is a chemical reaction where an aldehyde or a ketone is reacted with a Wittig Reagent (triphenyl phosphonium ylide) to give an alkene selectively and predictably. Such a reaction in organic chemistry is an important chemical reaction named after its discoverer, the German chemist Georg Wittig.
How are alkenes prepared from alcohol?
Alkenes or olefins can be prepared from primary alcohols by heating at 160−170 °C with concentrated sulfuric acid. For example, ethylene forms from ethanol.
CH3CH2OH (ethanol) → CH2=CH2 (ethylene)
Generally, acid-catalyzed dehydration of primary alcohol gives one alkene. However, secondary and tertiary alcohols give mixtures of alkenes due to the rearrangement of the carbonation intermediate. The rearrangement can be avoided when dehydrating by heated alumina.