Krypton Element
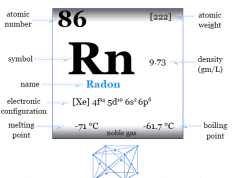
Krypton is a chemical element, inert gas, or noble gas of Group 18 of the periodic table with the symbol Kr and atomic number 36. It occurs in trace amounts in the earth’s environment and is used with other rare gases in fluorescent lamps. Krypton is a colorless, odorless, tasteless, monoatomic gas that forms a few numbers of chemical compounds with fluorine. Due to the filled valence orbital, the oxidation number or state is zero, but with fluorine, krypton also shows a +2 oxidation state. It is placed in the p-block of the periodic table with group members helium, neon, argon, xenon, and radon. The electromagnetic spectrum analysis of Krypton is characterized by several sharp emissions of green and yellow lines in the spectrum. In solid-state, it forms a face-centered cubic crystal lattice, and the cubic close-packed structure is the most space-economizing.
Discovery of Krypton
Krypton was discovered in 1898 by the British chemists Sir William Ramsay and Morris W. Travers and the name of the gas was derived from the Greek letter kryptos meaning hidden.
Properties of Krypton
The rare gas, Kr has valence shell electron configuration [Ar] 3d10 4s2 3p6, and heat capacity ratio (Cp/Cv) close to 1.66. It occurs in the earth’s atmosphere to the extent of 0.0001 percent. Therefore, it is obtained by low-temperature distillation of liquid air.
| Krypton | ||||
| Symbol | Kr | |||
| Discovery | Sir William Ramsay and Morris Travers in 1898 | |||
| Name derived from | Greek word kryptos, meaning hidden | |||
| Common isotope | 84Kr | |||
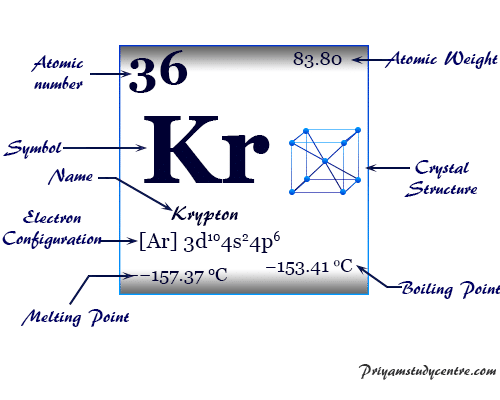
| Crystal structure | Face-centered cubic crystal lattice | |||
| Periodic properties | ||||
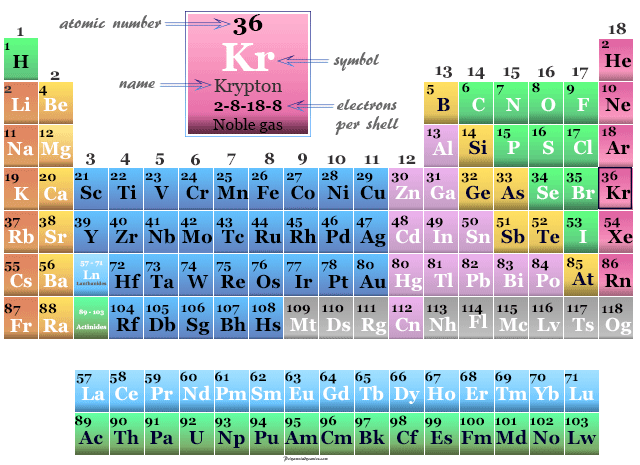
| Atomic number | 36 | |||
| Electron per shell | 2, 8, 18, 8 | |||
| Atomic weight | 83.798 | |||
| Electronic configuration | [Ar] 3d10 4s2 4p6 | |||
| Group | 18 (Noble gas) | |||
| Period | 4 | |||
| Block | p-block | |||
| Physical properties | ||||
| State at 20 °C | Gas | |||
| Melting point | −157.37°C, −251.27°F, 115.78 K | |||
| Boiling Point | −153.41°C, −244.15°F, 119.735 K | |||
| Density | 0.003425 g cm−3 | |||
| Chemical properties | ||||
| Atomic radius (non-bonded) | 2.02 Å | |||
| Covalent radius | 1.16 Å | |||
| Oxidation number or states | 0 | |||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd | |
| 1350.76 | 2350.37 | 3565.13 | ||
| Electron affinity | Unknown | |||
| Electronegativity | 3.00 (Pauling scale) | |||
| Molar heat capacity |
20.95 J mol−1 K−1 | |||
| CAS number | 7439-90-9 | |||
Isotopes of Kr
Naturally occurring krypton has six isotopes 84Kr (57 percent), 86Kr (17.3 percent), 82Kr (11.6 percent), 83Kr (11.5 percent), 80Kr (2.25 percent), and 78Kr (0.35 percent). It also formed about thirty-six unstable radioactive isotopes. These radioactive isotopes are obtained by nuclear fission of uranium or any other nuclear reaction.
- 78Kr (half-life 9.2×1021 years) is a stable isotope among all isotopes formed by radioactive decay reactions.
- 85Kr (half-life 10.76 years) is prepared normally by nuclear fission of uranium and plutonium. It is also emitted during the reprocessing of nuclear power reactors.
- The radioactive isotopes 81Kr are used by dating ground water.
Chemical Compounds
The chemistry of krypton or other noble gases like helium, neon, and argon is different from other periodic table elements. This is due to their filled valence shell configuration and high ionization energy.
However, it formed a few unstable chemical compounds with fluorine along with clathrates or cage compounds. The clathrates or cage compounds are formed mainly with water and para-quinol by trapping krypton molecules in the hydrogen bonding network.
Krypton Difluoride
The only known unstable halide compound is krypton difluoride (KrF2). The rare gas compound, KrF2 may be prepared by passing an electric discharge through a mixture of krypton and fluorine molecules at low pressure in a U-tube immersed with liquid oxygen. It is also formed by the action of OF2 on Kr in the presence of sunlight.
The chemical compound KrF2 is less stable than XeF2 and dissociated into Kr and F2 at room temperature. KrF2 uses a fluorinating agent that fluorinates xenon or noble metals like silver and gold.
A mixture of KrF2 and XeF2 fluorinated many trivalent lanthanide compounds like LnF4, LnF7−3, and LnOF2, where Ln = Ce, Pr, Nd, Tb, and Dy.
Krypton Difluoride Structure
A liner KrF2 has a similar structure to that of XeF2 with a Kr-F bond distance of 189 pm. However, the Kr-F bond energy is about 50 kJ mol−1. Such value is the lowest value of bond energy for chemical bonding formed by any element with fluorine.
It bonded to nitrogen in the salt, HCHKrF2+[AsF6]−. It is made by reacting KrF2 with HCN+[AsF6]− in a hydrogen fluoride solution.
Other nitrile adducts of krypton are also known, for example [RC≡NKrF]+, where R = Me, CF3, C2F5, and n-C3F5. Kr-O bond also presents in the thermally unstable compound like Kr(OTeF5)2.
Uses of Krypton

- Krypton is widely used in electronics like fluorescent lamps, and tubes. When a passage of electricity passes through a glass tube containing krypton, it emits a bluish-white light. Such a type of light is widely used in flash lamps for high-speed photography.
- It is also mixed with mercury to make the glow of bright greenish-blue light.
- In energy efficient fluorescent lamps, we used a mixture of krypton and argon to save power.
- The unstable chemical compound, krypton fluoride (KrF2) is also used in lasers for different types of experiments on nuclear fusion.
- Krypton and its isotopes are also used in different types of research in particle physics and chemistry, magnetic resonance imaging, and nuclear medicine.