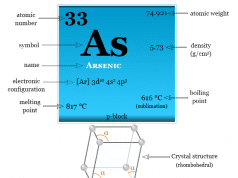
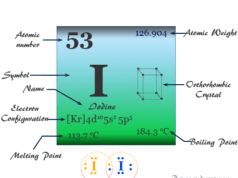
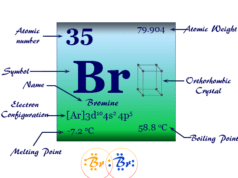
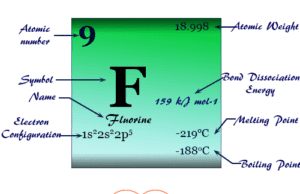
Phosphorus Element
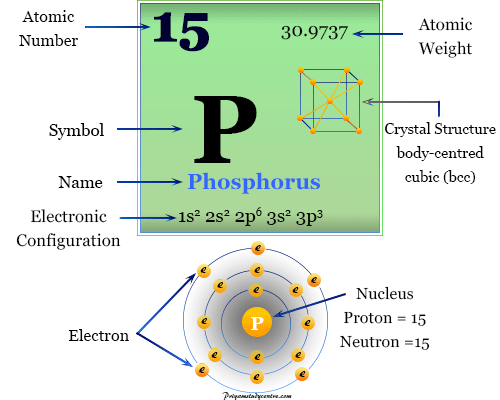
Phosphorus is a nonmetallic chemical element of the nitrogen family or Group 15 of the periodic table with the symbol P and atomic number 15. They are collectively called pnictogen or pnictides from the Greek word choking. Phosphorus is a colourless, semitransparent, soft, waxy solid at room temperature that glows in dark conditions. Nitrogen and phosphorus are essential constituents of the living cells. Another heavier member of the nitrogen family, arsenic is extremely toxic and causes water pollution. The chemistry of nitrogen and phosphorus differs, in the same way as silicon differs from carbon due to the presence of an empty d-orbital. The electronic configuration of phosphorus is closer to the next noble gas than the preceding noble gas. The phosphorus atom has 15 outer orbitals electrons and the nucleus contains 15 protons and 15 neutrons. The electrons are distributed as 2-8-5.
Properties of Phosphorus
Elemental phosphorus has several allotropic forms like white, red, violet, and black. In the liquid and gaseous state below 800 °C, it generally constitutes a tetrahedral P4 molecule. But above 800 °C, it forms a P2 molecule.
| Phosphorus |
||||
| Symbol | P | |||
| Discovery | Hennig Brandt in 1669 | |||
| Name derived from | The Greek word phosphoros, meaning bringer of light | |||
| Allotropes | White P, Red P, Black P | |||
| Common isotope | 31P | |||
| Crystal structure | Body-centered cubic (bcc) crystal lattice | |||
| Periodic properties | ||||
| Atomic number | 15 | |||
| Electron per shell | 2, 8, 5 | |||
| Atomic weight | 30.9737 | |||
| Electronic configuration | 1s2 2s2 2p6 3s2 2p3 | |||
| Group | group-15 | |||
| Period | period-3 | |||
| Block | p-block | |||
| Physical properties | ||||
| State at 20 °C | Solid | |||
| Melting point | white | red | ||
| 44.15 °C | 590 °C | |||
| Boiling Point | white: 280.5 °C | |||
| Density (g/cm3) | white | red | violet | black |
| 1.823 | 2.34 | 2.36 | 2.39 | |
| Chemical properties | ||||
| Atomic radius (non-bonded) | 1.80 Å | |||
| Covalent radius | 1.09 Å | |||
| Oxidation number or states | −3, −2, −1, 0, +3, +4, +4, +5 | |||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd | |
| 1011.8 | 1907 | 2914.1 | ||
| Electron affinity | 72.037 kJ mol−1 | |||
| Electronegativity | 2.19 (Pauling scale) | |||
| Molar heat capacity |
For white P: 23.824 J mol−1 K−1 | |||
| CAS number | 7723-14-0 (red) 12185-10-3 (white) |
|||
Phosphorus in the Periodic Table
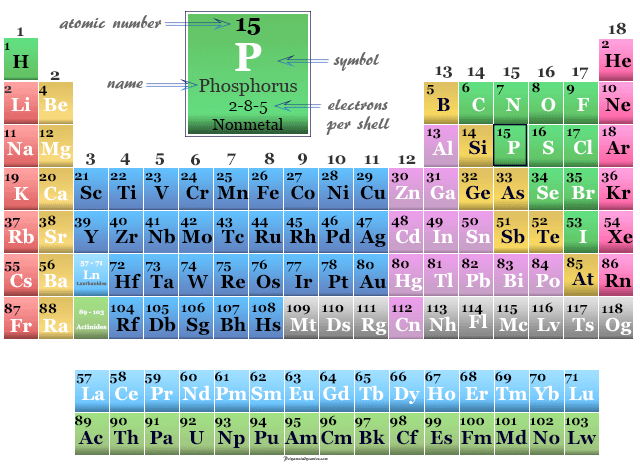
The valence shell electronic configuration of the element is 3s23p3 with two electrons in the s-orbital and three electrons in three p-orbitals. The electronic configuration suggests that phosphorus is a nonmetal or p-block element placed in group 15 of the periodic table.
Where is Phosphorus Found?
Phosphorus is a highly abundant chemical element in crustal rocks (1120 ppm). It is not found free in nature but is widely distributed in many minerals like fluorapatite, 3Ca3(PO4)2, and CaF2. It is also found in the plant and animal bodies where it is an essential element for many biological systems.
Over 200 phosphate minerals are distributed in different countries of the world. Morocco, the United States, Australia, China, and Russia are the major depositors of phosphorus minerals.
Isotopes of Phosphorus
It has 23 isotopes with mass numbers ranging from 25P to 47P. 31P is the only stable isotope of the element. 32P (beta ray emitter, t½ = 14 days) is the most important radioactive isotope obtained by (n,p) reaction on 32S or (n, γ) nuclear reaction on 31P in reactors. The radioactive decay of isotopes is extensively used in tracer studies.
Production Process
It is obtained during the reduction of phosphate rocks with carbon in the presence of sand at 1400 to 1500 °C in an electric furnace. The overall reaction may be,
2 Ca3(PO4)2 + 6 SiO2 + 10 C → 6 CaSiO3 + 10 CO + P4
The vapour is condensed under water molecules to form white phosphorus.
Allotropes of Phosphorus
Elemental phosphorus has several allotropic forms. White, red, violet, and black are the main allotropes of the element.
White Phosphorus
- White phosphorus is the most common allotrope with a melting point of 44 °C and a boiling point of 287 °C. It becomes yellow on exposure to light.
- It is insoluble in water but highly soluble in carbon disulfide, benzene, liquid sulfur dioxide, liquid ammonia, etc.
- Concentrated nitric acid slowly oxidizes the P4 molecule to form phosphoric acid.
- It is highly poisonous and contacts on skin or inhalation which may cause harmful metabolic disorders.
Red Phosphorus
- Red phosphorus is obtained by getting white-P in the absence of air at 270 °C to 300 °C.
- It is an amorphous solid, melting under pressure at a temperature of 592 °C.
- The nonpoisonous red phosphorus is much denser than the white allotrope.
- The reactivity of the red-P molecule is much less and unattacked by aqueous alkali metals.
Black Phosphorus
- Black phosphorus is the least reactive allotrope of the element. It is formed during heating the P4 molecule at about 200 °C and at very high pressure.
- An orthorhombic form is obtained at 1200 atmosphere pressure, still higher pressure forms rhombohedral and cubic crystal lattice are formed.
- It does not ignite in the air even at 400 °C and is thermodynamically the most stable allotrope of the element.
Chemical Compounds
The formation of a +5 cation by losing five outer electrons is just impossible. A huge amount of energy is necessary for this purpose. The fact suggests that the sum of five ionization energies cannot be compensated by gaining lattice energy for ionic bonding. Therefore most of the compounds in the P(V) are covalent in nature.
Hydrides of Phosphorus
Phosphine is the most common hydride of phosphorus with the chemical formula PH3. It is a volatile hydride formed by sp3 hybridization. Phosphine can be obtained during hydrolysis of metal phosphide. The thermal stability of the group-15 hydrides decreases rapidly with the increasing atomic weight of the parent element. The hydrides are less stable than the group-14 hydrides.
Phosphorus Halides
Due to the presence of vacant d-orbital, phosphorus formed two types of halides trihalides (PX3) and pentahalides (PX5). All the covalent trihalides (PX3) have tetrahedral (sp3) structures with a lone pair of electrons residing in the fourth orbital of the atom.
However, the pentahalides contain a trigonal bipyramidal structure with sp3d2 hybridization and 10 valence shell electrons. The pentahalide like PF5 is a very strong Lewis acid, forming complex compounds with amines or ethers. The valence shell of the element also expands in octahedral complexes like [PCl6]− that contain 12 electrons with sp3d2 hybridization.
Phosphorus Oxides
The elements of group 15 elements form oxides in their usual +3 and +5 oxidation states. Phosphorus generally forms two oxides P4O6 ( tetraphosphorus hexaoxide) and P4O10 (tetraphosphorus decaoxide) in the +3 and +5 oxidation states. It is readily oxidized to form P4O10 when burnt in excess air.
The structure of P4O10 is based on PO4 tetrahedra. P(III) oxide is formed when the element is burnt in a limited supply of oxygen. It is structurally similar to that of the P4 tetrahedron. It also formed a series of oxides P4Ox (x = 7, 8, 9).
Oxoacids of Phosphorus
Oxoacids of this element are extensively used and commercially important chemical or biological compounds. However, they are structurally complicated chemicals. They are acidic protons bound to oxygen atoms, some have nonacidic protons that are bonded directly to phosphorus and some contain P-P chemical bonding.
Only one is commercially important, and three of them are hypophosphorous acid, phosphorous acid, and phosphoric acid. Phosphoric acid (H3PO4) contains a tetrahedral skeleton with sp3 hybridization.
| Acids | Properties of oxoacids |
| Phosphonic acid (H3PO2) | It is a monobasic reducing agent known only in the solution. |
| Phosphorus acid (H3PO3) | It is a dibasic acid crystalline solid. |
| Hypophosphoric acid (H4P2O6) | It is a tetrabasic acid, although the known salts are Na2H2P2O6. |
| Phosphoric acid (H3PO4) | It is a tribasic acid with a very low melting point and a variety of salts of phosphoric acid is known. |
Uses of Phosphorus
- Phosphorus is the essential constituent of plants or animals and is used mostly for the preparation of concentrated phosphoric acid and phosphate fertilizer.
- It is converted to P4S10 and P4S3 which are used in the production of organo-phosphorus compounds and matches.
- Ferrophosphorus obtained as a byproduct in phosphorus preparation is used in high-density concrete, in radiation shields for nuclear power reactors, and in making special steel and cast iron.
- Phosphates generally enter the plant and animal kingdom through food chains and play a vital role in the formation of nucleic acids, metabolic functions via adenosine triphosphate (ATP), and the formation of bone, teeth, etc.
- A huge amount of phosphorus is also used in different types of detergent preparation.