Sodium Chloride Salt
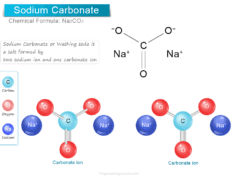
Sodium chloride (formula NaCl) is an essential inorganic salt which uses mainly to maintain blood pressure and balance our body fluids. It is found in most tissues and body fluids such as blood, sweat, and tears. Sodium chloride commonly called table salt uses widely in food processing, cosmetics, and skincare products. The use of sodium chloride in the chemical industry is simply numerous. NaCl is the starting material for the production of various sodium and chlorine compounds. It is used mainly for the production of sodium hydroxide (NaOH), sodium carbonate (Na2CO3), sodium sulfate (Na2SO4), hydrogen, chlorine, hydrochloric acid, etc. Sodium chloride dissolves in water to form a saline solution with pH ≈7. It is an ionic compound formed by the ionic bonding of sodium and chlorine atoms.
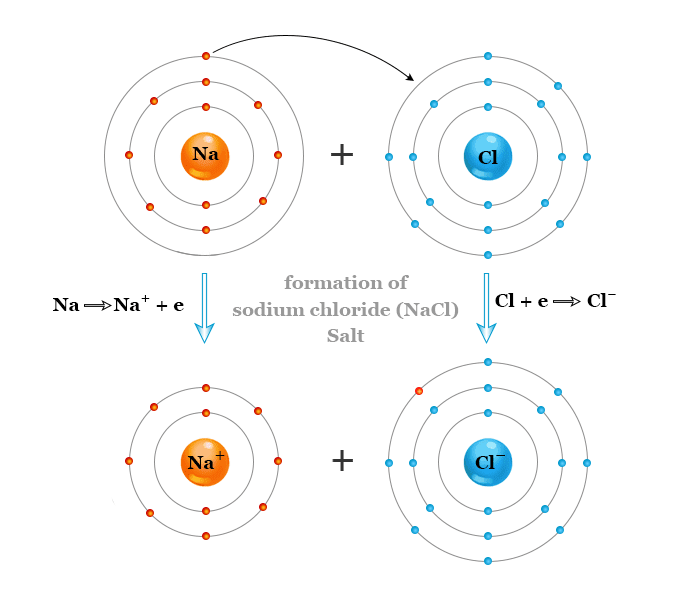
Each sodium atom loses one electron to form a unipositive sodium ion (Na+). Similarly, the chlorine atom gains one electron to form a uni negative chloride ion. The two oppositely ions Na+ and Cl− are held together by ionic bonding to form crystalline sodium chloride salt.
NaCl Crystal
Naturally, it occurs in seawater and mineral halite. Rock salt or sodium chloride is an ionic compound that forms a white crystalline solid.
NaCl crystal belongs to a face-centered cubic crystal lattice. The arrangement of Na+ and Cl− ions in NaCl crystal is given below the picture,
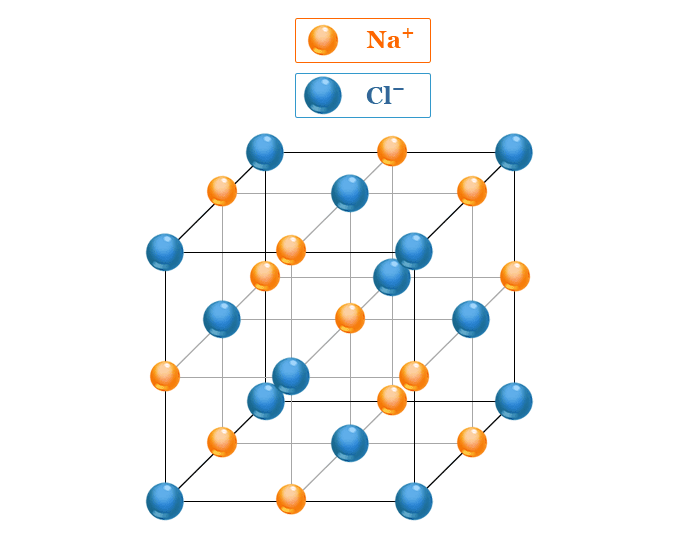
The two ions Na+ and Cl− then build up a packed type ionic crystal structure. The above picture shows that Cl− ions are located in the corner of the cube and center of the six faces while Na+ ions are located halfway between two chloride ions
Each sodium ion in NaCl crystal is shared equally by six chloride ions and each chloride ion by six sodium ions. The packing of two ions is repeated throughout in a non-ending pattern.
Properties
Sodium chloride is a white crystalline solid with a molar mass of 58.443 g. It is named common salt, rock salt, table salt, regular salt, sea salt, halite, and saline. NaCl provides flavors in food and is used as a binder and stabilizer.
It is soluble in water, ammonia, methanol, etc. The aqueous solution of sodium chloride is a good conductor of electricity due to the free movement of Na+ and Cl− ions.
Some common properties of NaCl are given below the table,
| IUPAC name | Sodium chloride |
| Other names | Common salt rock salt table salt regular salt sea salt halite saline |
| Chemical formula | NaCl |
| CAS Number | 7647-14-5 |
| Molar mass | 58.443 g/mol |
| Appearance | White crystalline solid |
| Melting point | 800.7 °C |
| Boiling point | 1,465 °C |
| Density | 2.17 g/cm3 |
| Solubility | Soluble in water, ammonia, methanol, etc |
| Crystal structure | Face-centered cubic crystal lattice |
Solid form
Crystallographic studies show that there is no discrete NaCl molecule. In solid NaCl, each Na+ ion is surrounded by six Cl− ions, or each Cl− ion is surrounded by six Na+ ions.
Sodium Chloride Solution
The seawater contains approximately 2.7-2.9% NaCl which is the major factor in the saline nature of seawater. It appears to be a white crystalline solid in pure form and dissolved in water to form a colorless solution.
The attraction between the Na+ and Cl− ions in the solid is very strong. Therefore, a highly polar solvent like water is used to make the NaCl solution. To make a saturated solution, we use 36 g of NaCl in 100 g of water at 293 K.
pH of Sodium Chloride Solution
The pH of aqueous sodium chloride solution ≈7. NaCl is formed from the neutralization reaction of a strong acid (HCl) and a strong base (NaOH). Therefore, when NaCl is mixed with water, the solution is neither basic nor acidic.
Sodium Chloride Uses
Annually, a million tonnes of sodium chloride salt is used for the production of many sodium and chlorine compounds. Paper, rubber, textiles, pharmaceuticals, skin care, water softening, and tanning industries are other chief consumers of sodium chloride salt.
It is used to make a wide range of domestic products such as plastic, paper, rubber, glass, chlorine, polyester, household bleach, soaps, detergents, and dyes.
Chemicals Production
Sodium chloride is an important inorganic raw material that uses for the production of various industrial chemicals.
- In the Chloralkali process, NaOH is the starting material for the production of chlorine and sodium hydroxide by electrolysis.
NaOH + H2O → Cl2 + H2 + 2 NaOH - It is converted to Na2CO3 and CaCl2 by the Solvay process.
- It is converted to sodium sulfate and hydrochloric acid by the Mannheim process.
These industrial chemicals are used widely in the Soda-ash industry for manufacturing glass, dyes, and many other domestic and commercial products.
Water Softening
Water softening is the process of removal of calcium, magnesium, and other metal cations from hard water. The resulting soft water does not build up insoluble scales or precipitates in household and industrial equipment and pipes. Hard water interferes cleaning process of soap.
Commercial and residential water-softening units use ion-exchange resins to remove calcium, magnesium, and other metal cations from hard water. These resins which use in the water softening system can generate and regenerated by NaCl or KCl salt.
Sodium Chloride Uses in Medicine
In medicine, we use different forms of sodium chloride such as injection, solutions, and drops. NaCl dissolves in water to form a saline solution.
FDA suggests that consuming too much NaCl salt can cause various health problems. It mainly causes high blood pressure or hypertension.
Intravenous or IV saline (0.9% NaCl) may be used to treat dehydration and electrolyte imbalance. It is also used in nasal sprays and eye drops.
Sodium Chloride in Skincare
Sodium chloride is a multifunctional ingredient for cosmetics and skincare products. It uses for the production of various oral hygiene products, shampoos, fragrances, skin, hair, nail, cleansing, makeup, and bath products.
According to U.S. Food and Drug Administration (FDA), the sodium chloride ingredient is safe to use.
- It is used in toothpaste and mouthwash products due to its ability to cleanse and deodorizes teeth and mouth. It also effectively polishes our teeth. Additionally, it imparts a flavor or a taste to such products.
- As a binder, NaCl crystal has properties to increase the thickness of the water portion of cosmetics and skincare products.
- In cosmetics and skincare, sodium chloride uses for the thickening and preservation of products.
Other Uses
- A major application of NaCl is de-icing of roadways in cold countries.
- It is used in the processing of various metals like aluminum, beryllium, copper, vanadium, and steel.
- The salt is used to bleach wood pulp in the pulp and paper industry.
- It also is used to make sodium chlorate. Sodium chlorate is added with sulfuric acid and water to form chlorine dioxide. Chlorine dioxide is an excellent oxygen-based bleaching agent.
- The salt is used to manufacture buna, neoprene, and white types of rubber.
- In the oil and gas industry, NaCl salt is an important component in the drilling process.
- The salt is used widely in dairy industries, meat packers, canning, baking, and grain mill products.
- Sodium chloride salt uses in the food industry as a flavor enhancer, preserver, binder, fermentation-control additive, texture-control agent, and color developer.