Europium Metal
Europium is a chemical element or rare earth metal in the periodic table with the symbol Eu and atomic number 63. It is the most reactive and softest rare earth metal that is stored under an inert fluid to protect it from atmospheric oxygen or moisture. It is an excellent absorber for neutrons and is widely used in control rods of nuclear power reactors. Europium is used as phosphors in euro currency notes, fluorescent lamps, color TV screens, computer screens, etc. It was found in most of the minerals containing the other rare elements.
Europium was first found by Paul Émile Lecoq de Boisbaudran but the credit for discovery was given to French chemist Eugène-Anatole Demarçay. He was able to isolate Eu in 1901. It is named after the continent of Europe.
Isotopes
Naturally occurring europium contains two isotopes 151Eu and 153Eu. The isotope Eu-151 is slightly more abundant but Eu-153 is more stable. It also contains 35 radioactive isotopes that can be prepared by various artificial nuclear reactions. The majority of these radioactive isotopes have half-lives shorter than 12.2 seconds.
The primary decay mode of europium isotopes is electron capture or beta decay. Like other lanthanides, various radioactive isotopes of Eu are produced during nuclear fission reactions.
Properties
Various properties of europium are strongly influenced by its half-filled electron configuration. The melting point and density of Eu are low among the lanthanides.
It is a ductile metal that adopts a body-centered cubic crystal lattice. The hardness of this rare earth metal is similar to that of lead.
| Europium | |||
| Symbol | Eu | ||
| Discovery | Eugène-Anatole Demarçay in 1901 | ||
| Name derived from | It is named after the continent of Europe | ||
| Common isotope | 63Eu153 | ||
| Oxidation states | +3, +2 | ||
| CAS number | 7440-53-1 | ||
| Periodic properties | |||
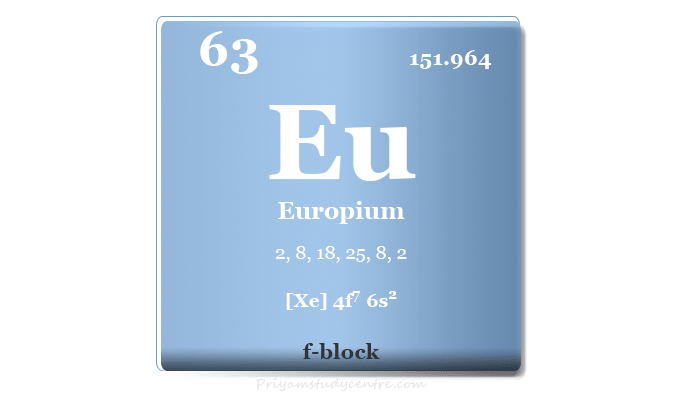
| Atomic number | 63 | ||
| Relative atomic mass | 151.964 | ||
| Electron per cell | 2, 8, 18, 25, 8, 2 | ||
| Electronic Configuration | [Xe] 4f7 6s2 | ||
| Block | f-block | ||
| Group | Lanthanides | ||
| Period | 6 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 822 °C, 1095 K | ||
| Boiling point | 1529 °C, 1802 K | ||
| Molar heat capacity | 27.66 J mol−1 K−1 | ||
| Crystal structure | body-centered cubic (bcc) | ||
| Density | 5.24 g/cm3 | ||
| Heat of fusion | 9.21 kJ mol−1 | ||
| Heat of vaporization | 176 kJ mol−1 | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.35 Å | ||
| Covalent radius | 1.83 Å | ||
| Electronegativity | Unknown | ||
| Electron affinity | 83.363 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 547.11 | 1085.46 | 2404.41 | |
Electron Configuration of Europium
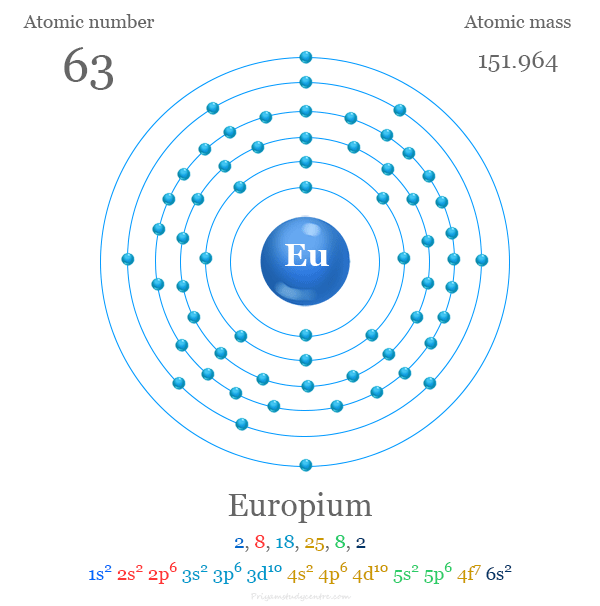
The 63 electrons of europium are arranged in the configuration of [Xe] 4f7 6s2. It loses two electrons to form a stable half-filled f7 configuration. Therefore, it shows a +2 oxidation state along with a +3 state.
Europium in the Periodic Table
The rare earth metal europium is placed in the f-block elements of the periodic table. It is a lanthanide that lies between samarium and gadolinium.
Chemical Properties
Europium is a highly electropositive and active rare earth metal. The reactivity of Eu with water is comparable to that of alkaline earth metals calcium.
2 Eu + 6 H2O → 2 Eu(OH)3 + 3 H2
The rare earth metal europium tarnishes slowly in the air by forming an oxide layer. Like other rare earth metals, it also burns in oxygen at 150 °C to form Eu (III) oxide.
4 Eu + 3 O2 → 2 Eu2O3
It is attacked by acids like dilute sulfuric acid with the liberation of hydrogen gas. The acidic solutions contain pale pink Eu3+ ions.
2 Eu + 3 H2SO4 + 18 H2O → 2 [Eu(H2O)9]3+ + 3 SO4−2 + 3 H2
Facts About Europium
- It is a ductile, lustrous lanthanide that shows a silvery appearance.
- It is one of the softest rare earth metals that can be cut by a knife.
- Europium is one of the rarest and most costly rare-earth metals that has a price of about $7500/kg.
- It is not found in nature as a free element and the most important sources of Eu are bastnäsite, monazite, and xenotime.
- Like other rare earth metals, it may also be separated by solvent extraction process or ion exchange chromatography.
- Like other rare earth metals, it shows a +3 oxidation number or state.
- The +2 oxidation number is common for europium due to the stable f7 configuration of the Eu+3 ion.
- Europium is a non-toxic metal that does not show any biological role in human beings.
- Europium is used in the printing of euro banknotes because it glows red under UV light. Therefore, forgeries can be detected by the lack of red glow in euro banknotes.
Chemical Compounds
Like other lanthanides, europium is usually trivalent but it readily forms divalent compounds. In the +2 state, the valence shell electron configuration of the Eu+2 ion is [Xe] 4f7. The half-filled f-shell provides more stability for the Eu+2 ion. The most common chemical compounds of europium are given below,
Oxides
It forms europium (III) and europium (II) oxides with the chemical formulas Eu2O3 and EuO. Eu (II) oxide may be prepared by the reduction of Eu (III) oxide with elemental europium at 800°C and subsequent vacuum distillation at 1150°C.
The oxide Eu2O3 is widely used as a red or blue phosphor in television sets and fluorescent lamps. Eu (II) oxide has semiconductor properties with a band gap of 1.12 eV.
Europium Hydroxide
Europium hydroxide is an inorganic compound with the chemical formula Eu(OH)3. It can be prepared by reacting metallic Eu with water. It decomposes to EuO(OH) at elevated temperatures but further decomposition produces Eu2O3.
Halides
The rare earth metal europium reacts with all the halogens like fluorine, chlorine, bromine, and iodine to form corresponding halides.
2 Eu + 3 X2 → 2 EuX3 (X = F, Cl, Br, I)
This route gives europium (III) halides like white fluoride (EuF3), yellow chloride (EuCl3), gray bromide (EuBr3), and colorless iodide (EuI3).
It also forms corresponding dihalides like yellow-green fluoride (EuF2), colorless chloride (EuCl2), colorless bromide (EuBr2), and green iodide (EuI2).
Sulfate
It forms Eu (III) and Eu (II) sulfates with the chemical formula Eu2(SO4)2 and EuSO4 respectively.
Europium nitrate
Eu (III) nitrate is a compound with the chemical formula Eu(NO3)3. It is obtained by dissolving Eu2O3 in dilute nitric acid.
The most common hexahydrate form is a colorless hygroscopic crystalline solid. It reacts with 1,3,5-trimesic acid to produce a coordination polymer under hydrothermal conditions.
Europium uses
- Europium is used in anti-counterfeiting phosphors in euro currency notes that glow red under ultraviolet-visible (UV) light.
- It is an excellent absorber for neutrons in control rods of nuclear power reactors.
- It is used for making thin superconducting alloys.
- It is also used for manufacturing fluorescent glass which increases the general efficiency of fluorescent lamps.
- Europium oxide is used for making color TV screens and computer screens.
- It is a dopped in some types of glasses for lasers and optoelectronic devices.
- In drug discovery, europium fluorescence is used to interrogate biomolecular interaction screens.