Nucleotide Synthesis
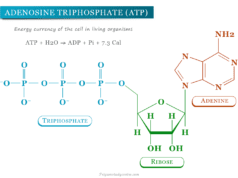
Nucleotides that contain a nitrogenous base, a pentose sugar, and a phosphate group in their structure are the basic building blocks of nucleic acids − deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). A nucleotide is an organic biopolymer that can be synthesis in our bodies and is also essential for our lives. Purine and pyrimidine nucleotides are the main structural components of DNA, RNA, and some coenzymes of B vitamins. Therefore, they are the monomeric units of nucleic acid (DNA and RNA) and coenzyme. Nucleotides can perform a wide variety of metabolic functions – energy metabolism, amino acid synthesis, protein synthesis, and controlling enzyme activities within the cells.
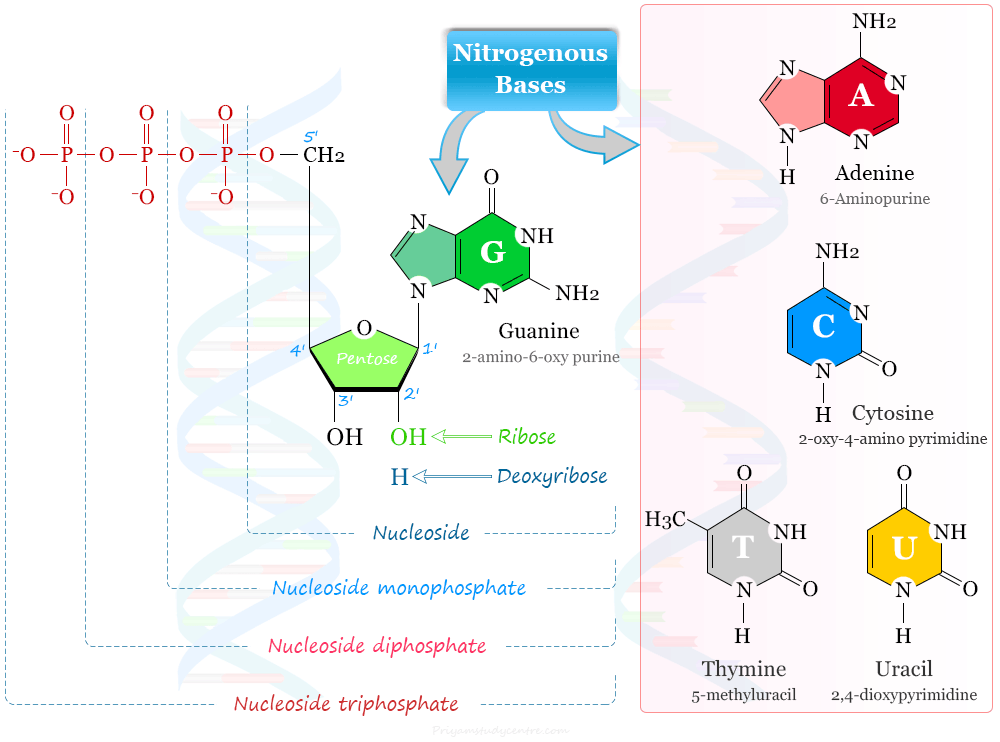
Nucleotide Structure
A nucleotide is a biomolecule that contains three units that are covalently linked to each other. The three units are
- Nitrogenous bases: Purine and Pyrimidine
- Pentose Sugar: Ribose and Deoxyribose
- Phosphate: monophosphate, diphosphate, or triphosphate
The atoms of the purine ring are numbered from 1 to 9 and for pyrimidine from 1 to 6. The pentose sugar is represented by 1′ to 6′.
Nitrogenous Bases
The nitrogenous bases found in nucleotides of DNA and RNA are two types−purines and pyrimidines. The nitrogenous bases purines are numbering in the anticlockwise direction while pyrimidines are numbering in the clockwise direction.
Purine Bases
Purines are the heterocyclic aromatic organic compound that contains a pyrimidine ring fused with an imidazole ring. Purine bases (guanine and adenine) form corresponding nucleotides deoxyguanylate and deoxyadenylate with deoxyribose and phosphate groups. These nucleotides are the basic building blocks of nucleic acids (DNA and RNA).
Pyrimidine Bases
Cytosine (C), thymine (T), and uracil (U) are three pyrimidine bases that are used in the biological process for the formation of DNA and RNA nucleotides. Pyrimidine is a heterocyclic organic compound that has a six-membered ring with two nitrogen atoms at positions 1 and 3 in the ring.
The nucleic acids (DNA and RNA) contain the same purine bases namely adenine (A) and guanine (G). Further, the pyrimidine base cytosine (C) is located in the nucleotide of DNA and RNA.
However, the nucleic acid differs with respect to the second pyrimidine base. DNA contains thymine (T) whereas RNA contains uracil (U) in its nucleotide. The thymine and uracil differ in structure by the presence or absence of a methyl group.
Pentose Sugar
A nucleotide of DNA (deoxyribonucleic acid) contains pentose sugar deoxyribose while RNA (ribonucleic acid) contains ribose sugar.
Ribose and deoxyribose differ from each other by the presence or absence of the hydroxyl group on the 2′ carbon. Ribose sugar has a hydroxyl group at 2′ carbon while deoxyribose does not contain such hydroxyl group at 2′ carbon.
The pentoses are bound to nitrogenous bases by glycosidic bonds. For example, the 9 position of a purine ring binds with 1′ position of a pentose sugar to form a covalent bond in the purine nucleoside. In the case of pyrimidine nucleosides, the glycosidic linkage is between the 1 position of pyrimidine and the 1′ position of a pentose sugar.
Phosphate Group
The phosphate group interlinks the sugar molecules of two nucleotides of DNA and RNA to form a chain. Therefore, polynucleotides and the phosphate chain form the backbone of polynucleotides of DNA and RNA.
Nucleotides in DNA
The DNA molecule contains two long-chain polymers of polynucleotide. DNA contains four types of nucleotide subunits deoxyadenylate (dAMP), deoxyguanylate (dGMP), and deoxycytidylate (dCMP) in its structure.
The nucleosides and nucleotides of DNA are given below in the table
| Nucleosides and nucleotides of DNA | |||
| Base | Deoxyribonucleoside | Deoxyribonucleotide | Abbreviation |
| Adenine | Deoxyadenosine | Deoxyadenosine 5′ monophosphate | dAMP |
| Guanine | Deoxyguanosine | Deoxyguanosine 5′ monophosphate | dGMP |
| Cytosine | Deoxycytidine | Deoxycytidine 5′ monophosphate | dCMP |
| Thymine | Deoxythymidine | Deoxythymidine 5′ monophosphate | dTMP |
Structure of DNA Nucleotide
The three-dimensional structure of DNA or the double helix comes from the chemical and structural features of its two polynucleotide chains held together by hydrogen bonding between the bases of the different strands. These types of hydrogen bonding of polynucleotide chains or DNA chains form complementary base pairs.
The A−T pair contains 2 hydrogen bonds while the G−C pair contains 3 hydrogen bonds. Therefore, hydrogen bonds are formed between purine and pyrimidine only.
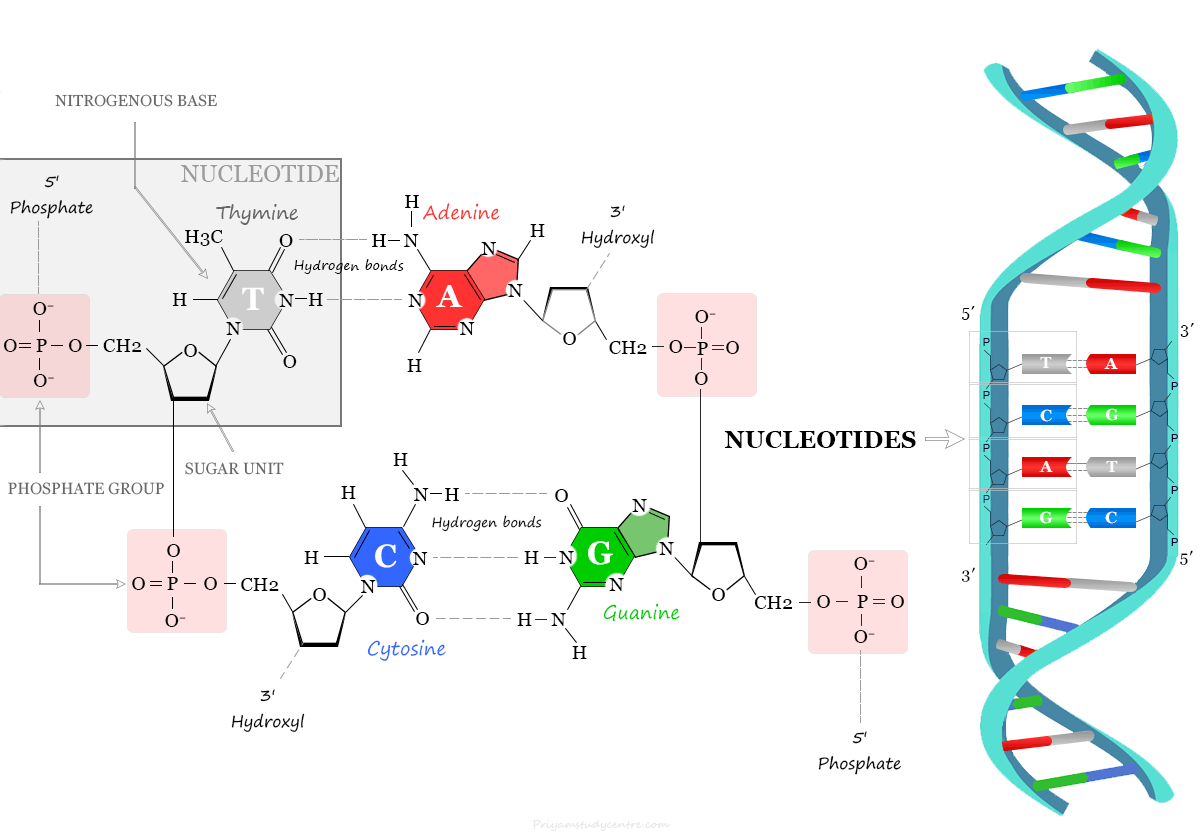
If two purine bases face each other, they do not fit into the allowable space, and two pyrimidine bases are too far to form hydrogen bonding. Therefore, the only base arrangement possible in DNA structure is A−T, T−A, G−C, and C−G. All the bases in DNA are present inside the double helix and the sugar-phosphate backbones are on the outside.
Nucleotides in RNA
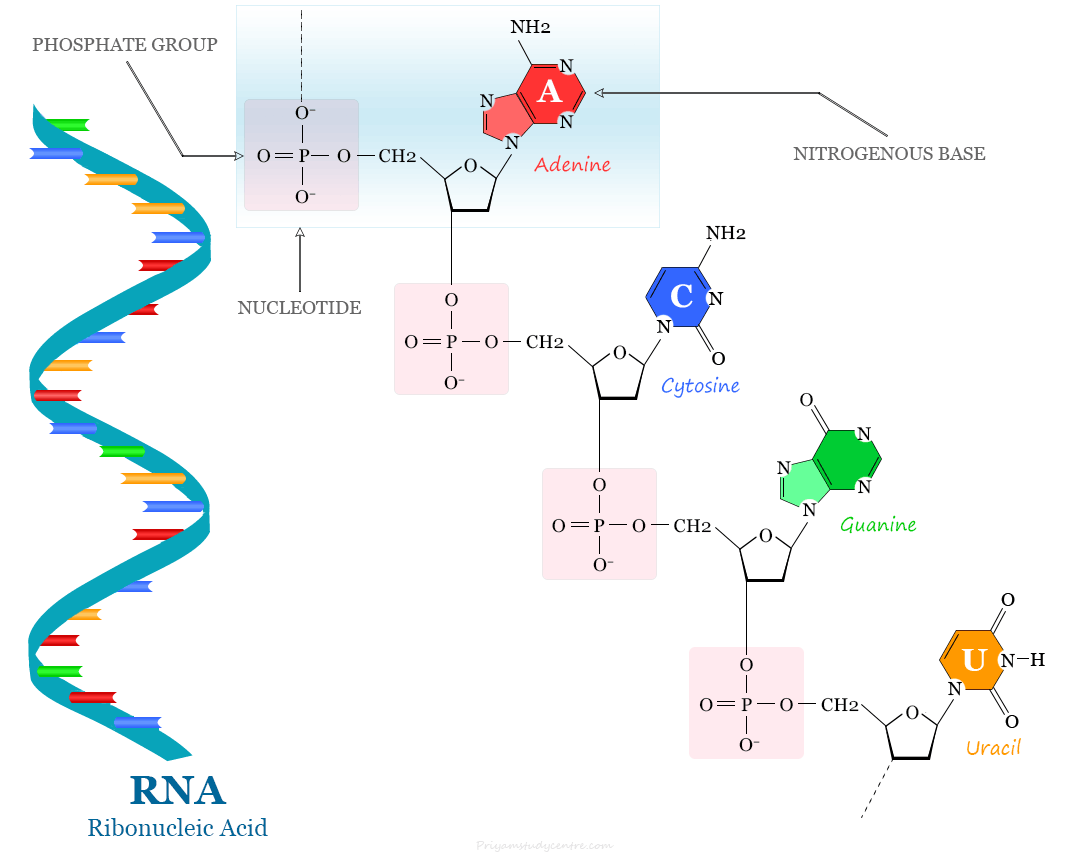
Ribonucleic acid (RNA) is a polymer of nucleotides held together by 3′,5’−phosphodiester bridges. RNA has certain similarities with DNA but they also show certain differences in their structure. RNA chemically differs from DNA in respect to pentose sugar and pyrimidine bases.
- Pentose: The sugar in RNA nucleotide is ribose in contrast to deoxyribose in DNA.
- Pyrimidine: RNA nucleotide contains the pyrimidine uracil in place of thymine.
The ribonucleosides and ribonucleotides of RNA are given below in the table,
| Nucleosides and nucleotides of DNA | |||
| Base | Ribonucleoside | Ribonucleotide | Abbreviation |
| Adenine | Adenosine | Adenosine 5′ monophosphate | AMP |
| Guanine | Guanosine | Guanosine 5′ monophosphate | GMP |
| Cytosine | Cytidine | Cytidine 5′ monophosphate | CMP |
| Uracil | Uridine | Uridine 5′ monophosphate | UMP |
Structure of RNA Nucleotide
A nucleotide of RNA is the basic building block of RNA structure. It contains a ribose sugar, a phosphate, and one of the four nitrogenous bases (adenine, guanine, cytosine, and uracil).
Most RNA molecules are single-stranded but base pairing via hydrogen bonds is the basis of the secondary structure of RNA molecule. Therefore, some RNA molecules may contain double-stranded regions formed by complementary base pairing.
For example, ribosomal RNA (rRNA) and transfer RNA (tRNA) exhibit double-stranded regions in their secondary structure.
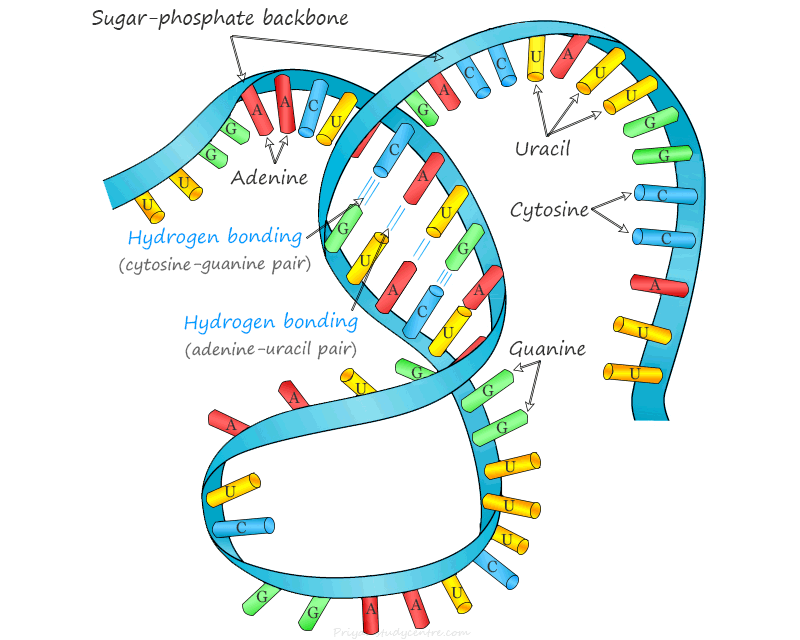
Two types of base pairing are possible in RNA secondary structure adenine and uracil (A-U) and cytosine and guanine (C-G). The adenine and uracil pair contain two hydrogen bonds while three bonds form between the cytosine and guanine pair.
Nucleoside to Nucleotide
The addition of pentose sugar to a nitrogenous base produces a nucleoside.
- If pentose sugar is ribose, ribonucleosides are formed. Adenosine, guanosine, cytidine, and uridine are ribonucleosides of adenine, guanine, cytosine, and uracil respectively.
- If pentose sugar is deoxyribose, deoxyribonucleosides are formed. Deoxyadenosine, deoxyguanosine, deoxycytidine, and deoxythymidine are deoxyribonucleosides of adenine, guanine, cytosine, and thymine respectively.
The term mononucleotide is used when a single phosphate moiety is added to nucleoside. Therefore, adenosine monophosphate (AMP) contains adenine + ribose + phosphate.
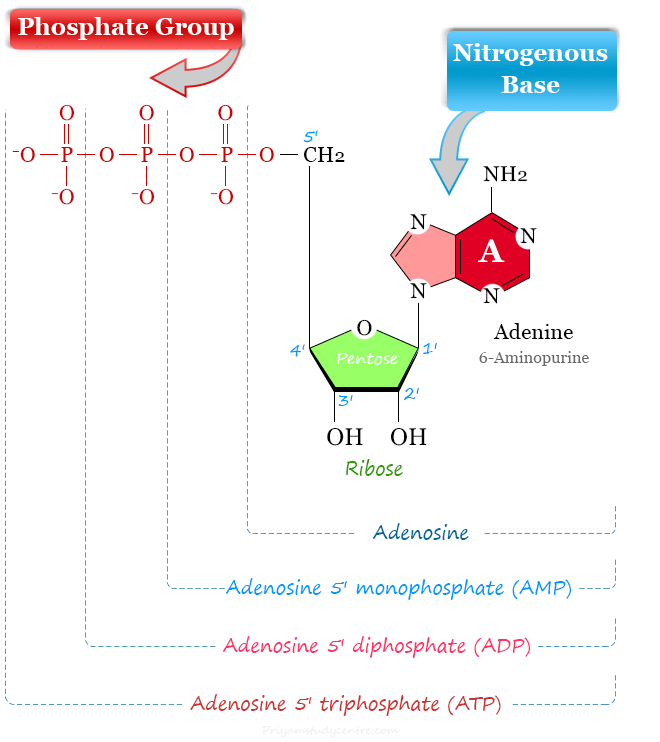
The terms dinucleotide and trinucleotide are used on the basis of a number of phosphate groups attached to the nucleoside. For example, adenosine diphosphate (ADP) and adenosine triphosphate (ATP) contain two and three phosphate groups on adenosine nucleoside.
Nucleotide Biosynthesis
Nucleotides are the monomers that contain a sugar, a phosphate, and a nitrogenous base. It polymerizes to produce nucleic acids (DNA and RNA). The bases present in nucleic acids are either purines or pyrimidines.
Humans can synthesize purine and pyrimidines to meet the variable demands of the body. Therefore, dietary nucleic acids and nucleotides are non-essential for the human body. These two types of nucleotides are primarily produced in the liver but the two different groups are synthesized in different ways.
The synthesis of nucleotide requires phosphoribosyl pyrophosphate (PRPP). It donates the ribose and phosphate group for the synthesis of nucleotides.
Synthesis of Purine Nucleotide
The liver is the major site for the synthesis of purine nucleotides in our bodies. Erythosites, polymorphonuclear leukocytes, and the brain cannot produce purines.
The most important steps for purine nucleotide synthesis are,
- Ribose 5-phosphate, produced in hexose monophosphate shunt of carbohydrate metabolism is the starting material for purine nucleotide synthesis. This ribose 5-phosphate reacts with adenosine triphosphate (ATP) to form phosphoribosyl pyrophosphate (PRPP).
- PRPP converted to inosine monophosphate (IMP) in the presence of glutamine, glycine, 5,10-methyltetrahydrofolate, aspartate, ATP, adenylosuccinate, etc. Inosine monophosphate (IMP) is the immediate precursor for the formation of nucleotides AMP and GMP.
- Aspartate condenses with IMP in the presence of GTP to produce adenylsuccinate which on cleavage forms nucleotide adenosine (AMP).
- For the synthesis of GMP, IMP undergoes NAD+-dependent dehydrogenation to form xanthosine monophosphate (XMP). Glutamine then transfers amide nitrogen to XMP to produce nucleotide GMP.
Synthesis of Pyrimidine Nucleotide
The synthesis of pyrimidine nucleotide is a much similar process compared to that of purine nucleotide. Aspartate, glutamine, and carbon dioxide contribute to atoms in the formation of the pyrimidine ring.
The pyrimidine ring is first synthesized and then attached to ribose 5-phosphate. It is in contrast to purine nucleotide synthesis wherein the purine ring is built upon a pre-existing ribose 5-phosphate. The synthesis of pyrimidine nucleotide follows the following steps−
- Glutamine transfers its amide nitrogen to CO2 and produces carbamyl phosphate. It is an ATP-dependent biochemical reaction catalyzed by the enzyme carbamoyl phosphate synthetase II (CPS-II).
- This carbamoyl phosphate condenses with aspartate to form carbamoyl aspartate.
- The next step in pyrimidine nucleotide synthesis is NAD+-dependent dehydrogenation, leading to the formation of orotate.
- Orotate now interacts with ribose 5-phosphate to produce orotidine monophosphate (OMP). This OMP undergoes decarboxylation to form uridine monophosphate (UMP).
- By an ATP-dependent kinase reaction, UMP is converted to UDP which is a precursor for the synthesis of dUMP, dTMP, UTP, and CTP.
Nucleotides Function
Nucleotides are essential components of living cells and perform a wide variety of biological functions within living organisms.
Other than the polynucleotide of DNA and RNA, nucleotides are present in our body in various forms and control various essential biological processes for life. These nucleotides are ATP, cAMP, NAD+, NADP+, FAD, coenzyme A, etc.
The most common biological function of such types of nucleotides are
- Purine and pyrimidine nucleotides are the basic building blocks of DNA and RNA which carry genetic information and participate in protein synthesis.
- Nucleotides play an essential role in energy metabolism, amino acid, protein, and cell membrane synthesis of living organisms. They provide chemical energy for these biological processes in the form of nucleoside triphosphates such as adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP), and uridine triphosphate (UTP).
- Nucleotides are important structural components of various coenzymes (FAD, FMN, NAD, and NADP+) and B-complex vitamins.
- NAD and NADP are two important nucleotides that carry electron/electrons for many biological redox reactions.
- Cyclic adenosine monophosphate (cAMP) and CDP-acylglycerol are important intermediates in carbohydrate and lipid metabolism.
- Various synthetic analogues of nucleotides are employed in the treatment of cancer, AIDS, and suppression of immune response during organ transplantation.
Nucleoside Analogues
The nucleoside analogues are an important class of antiviral agents that are very useful in clinical medicine. They are commonly used against,
- HIV infection
- Hepatitis B virus (HBV)
- Hepatitis C virus (HCV)
- Cytomegalovirus (CMV)
- Herpes simplex virus (HSV)
- Varicella-zoster (VZV) infection
Nucleotide analogues are nucleotides that contain a nucleic acid analogue, a sugar unit, and a phosphate group with one to three phosphates. The pharmacological applications of various nucleoside analogs are,
- Allopurinol is a xanthine oxidase inhibitor used in the treatment of hyperuricemia and gout.
- Zidovudine and didanosine are sugar-modified synthetic analogues used for the treatment of AIDS.
- 5-Fluorouracil, 6-mercaptopurine, 8-aza-guanine, 3-deoxyuridine, 6-azauridine, 6-azacytidine, and 5-iodouracil are analogue used in treating cancer. These compounds can block cell proliferation.
- The nucleotide analogue azathioprine is used to suppress immunological rejection during kidney transplantation or organ transplantation.
- Arabinosylcytosine is a synthetic nucleotide analogue that interferes with DNA replication and is used in cancer therapy.
Frequently Asked Questions
Which nucleotide component contains nitrogen?
Nucleotides contain a nucleotide base, a pentose sugar, and one to three phosphate groups. All nucleotide bases are nitrogen-containing derivatives of heterocyclic compounds – pyrimidine (cytosine, thymine, and uracil), purine (adenine and guanine), and pyridine (nicotinamide).
How many nucleotides in DNA?
Each DNA strand is composed of four different nucleotides linked end to end to form a long-chain polymeric structure. These four nucleotides are deoxyadenylate, deoxyguanylate, deoxycytidylate, and deoxythymidylate.
Which nucleotide is required for glycogen synthesis?
Uridine triphosphate (UTP) is a nucleotide required for glycogen synthesis. When UDP-glucose phosphorylase combines the nucleotide uridine triphosphate (UTP) and glucose-1-phosphate, it forms pyrophosphate and UDP-glucose, the immediate precursor of glycogen synthesis.
How many nucleotides make up a codon?
A sequence of three nucleotides in messenger RNA (mRNA) makes a codon for a particular amino acid.
The codon or genetic code is regarded as a directory of nucleotides that determines the sequence of amino acids in proteins. Sixty-one codons code for twenty amino acids found in protein.
Where are nucleotides located?
The nucleotides are the structural components of nucleic acids (DNA and RNA) and coenzymes that are essential biomolecules located in all life-forms on life.
Nucleotide is obtained from diet but metabolizes to form uric acid. Humans can synthesize purines and pyrimidine nucleotides to meet the variable demands for DNA and RNA synthesis.