Articles About Chemistry
Articles about chemistry in online free chemistry courses at Priyamstudycentre provide a basic knowledge or study guide to learn chemistry articles or topics for all school, college, and university students.
During the last few years, the chemistry syllabus of the different regions of the world has been trying to modernize. Hence the syllabus of chemistry changed. Therefore, this change results in a shortage of suitable texts for many schools, colleges, and university students. The authors of Learning Chemistry seek this change and try to fill this gap.
- This Page is designed to incorporate the latest recommendations in the field of chemistry.
- All the posts, pages, and applications of principles are published in the form of solved problems and numerical online quizzes.
- Posts and pages published in this domain have proper headings to understand the text more systematically.
- All posts and pages are updated regularly and the labels give for this updating.
The author wishes to thank all those who helped in the presentation of the posts and pages. Since drawbacks and better methods of presentation are always there.
Suggestions for improvement and criticism will be gratefully received and acknowledged. For any improvement please contact us online at chemistry@priyamstudycentre.com.
How to Learn About Chemistry?
In recent years teaching classes of chemistry in many schools, colleges, and universities have changed. The syllabus of education is based on a theoretical and conceptual method and the application of the basic concepts.
We observed this change in chemistry classes and frightened undergraduate students whose performance in chemistry is far from satisfactory. This poor performance is partly because of the non-availability of comprehensive notes. This stresses the logical deduction and the solution of numerical. The students find themselves unduly studying materials.
We published some posts and pages which cover the theory as well as the application of this study materials. Online learning school college chemistry courses are primarily intended for our readers to find chemistry topics more easily.
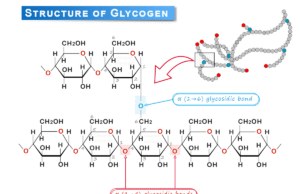
States of Matter in Chemistry
Crystalline solids or amorphous solids are characterized by their high density and low compressibility compared with those of the gas phase. This part provides different topics related to solid and gaseous-state chemistry such as
- cubic crystal lattice
- properties of gases
- surface tension
- Graham’s law of diffusion
- ideal gas law
- Van der Waals equation
In order to determine experimentally the properties of substances, we deal with the aggregates of molecules as they occur in the environment.
Various kinds of substances that make up matter are divided into three main categories,
- Gases
- Solids
- Liquids
These states of matter are interchangeable. For example, below the critical temperature, the gas is liquefied to form a solid or the specific heat solid is converted into a liquid state.
Chemical Kinetics and Equilibrium
In this part, we can deal with the chemical equilibrium of a chemical reaction such as homogenous and heterogeneous.
The main related topics are,
- Mass action law
- Vant Hoff equation
- Le Chatelier’s principle
- Thermodynamics conservation of energy
- Free energy
- Internal energy
- Entropy
- Enthalpy
To know the speed of a chemical reaction we need to know about how much time is needed to complete the chemical reaction. Chemical kinetics and chemical catalyst are essential parts of chemistry to determine the speed of zero-order kinetics, first-order, or second-order chemical reactions.
Covalent Bond Polarity
A polar molecule is one in which there will be a separation of the center of gravity of the positive charge and negative charges. Bonds Polarity developed a positive and negative pole in the chemical bond.
In an electric field, the polar molecule tends to align themselves with the positive ends facing the negative pole of the electric field but the negative ends face the positive pole. Hence the electric polarization of molecules is expressed by the dipole moment.