Solvent
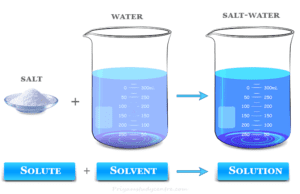
Solvent Definition in Chemistry
A solvent in chemistry is a chemical substance in which other types of materials dissolve to form a solution. It is...
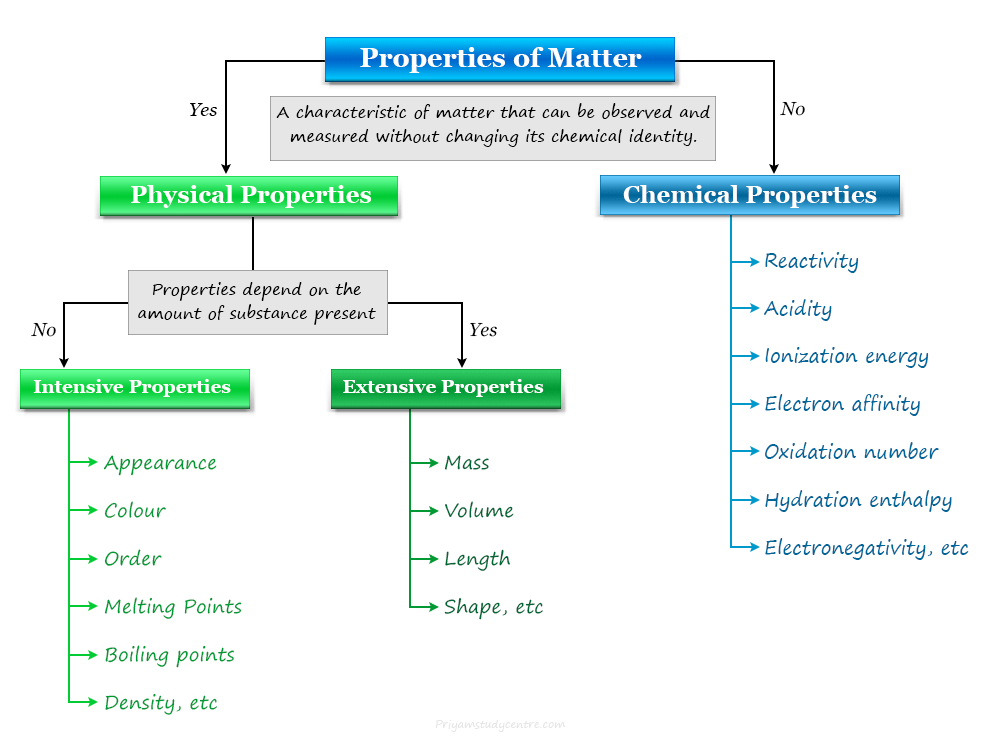
Solution
Solution Definition in Chemistry
A solution in chemistry is a type of homogeneous mixture of two or more chemical substances where a minor component (solute)...
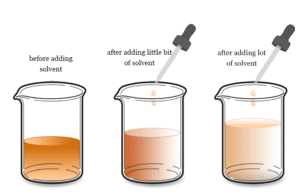
Concentration
Concentration of a Solution
Concentration in chemistry is a calculation of how much substance is present in a chemical solution or mixture. In learning chemistry, the...
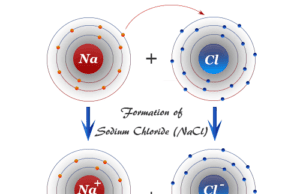
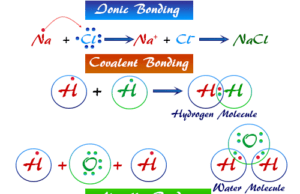
Ionic Bonding
Ionic Bonding Definition
Ionic bonding or ionic bond is the electrostatic force that binds together oppositely charged ions formed by the transfer of electron or...
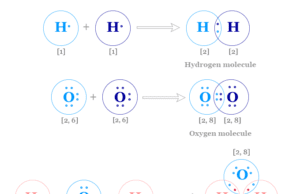
Covalent Bond
Covalent Bond Examples
Covalent bond or covalent bonding may be defined as a chemical bond that is formed by the sharing of one or more...
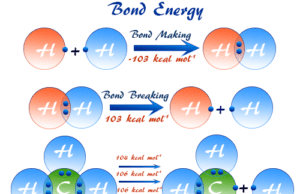
Bond Energy
Bond Energy and Dissociation Energy
Bond energy or bond enthalpy is the average value of the dissociation energies of a given bond in a series...
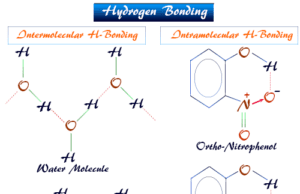
Hydrogen Bonding
Hydrogen Bonding Examples
Hydrogen bonding or intermolecular and intramolecular hydrogen bond is a weak type of chemical bond due to very unstable attractive forces responsible...
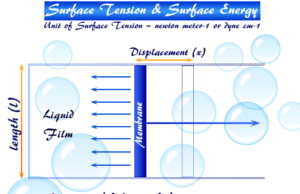
Surface Tension
Surface Tension of Liquid
Surface tension or surface energy is the most important characteristic property of liquid origin at the surfaces and is displayed when...
Chemical Bonding
Chemical Bonding in Chemistry
Chemical bonding or chemical bond in learning chemistry is the different types of forces that bind together two common atoms or...
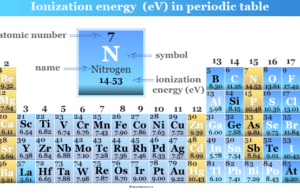
Ionization Energy
Ionization Energy in Periodic Table
Ionization energy or ionization potential in chemistry is the minimum amount of energy required to remove the outer electron of...