Chemistry Test Online
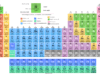
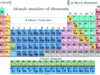
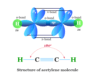
Chemistry test online practice questions answers for high school or college grade chemistry students to help in medical (NEET) and engineering (JEE, IIT) examinations. It contains multiple-choice questions (MCQs) and answers based on chemistry principles, concepts, formulas, mathematical problems, etc. Chemistry tests provide fully solved questions and answers useful for competitive exams like NEET, JEE, IIT, IBPS, SBI, SSC, RRB, GATE, etc. It also provides various biology or biochemistry questions and answers in the form of MCQ for medical exams. Therefore, start these online quizzes in learning chemistry and evaluate yourself or improve your knowledge of chemistry.