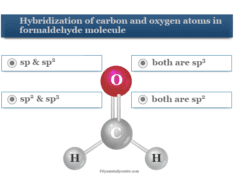
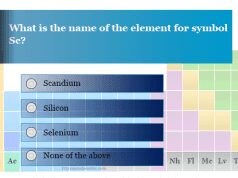
Chemistry Quiz 1 Online Practice Test
Chemistry Quiz 1 or online practice test contains ten chemistry quiz questions with answers for 9, 10, 11, and 12 grade or class science students. The online chemistry quiz in learning chemistry contains multiple choice questions (MCQ) which are asked in various classes and competitive examinations.
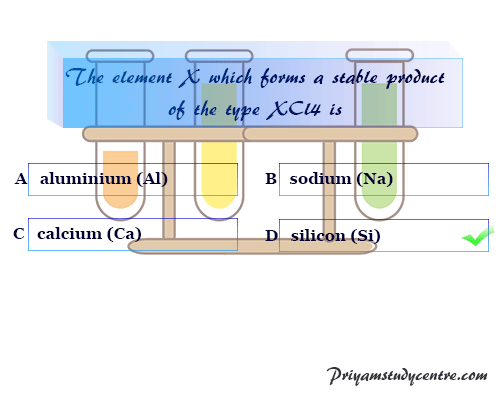
The chemistry study materials and MCQ questions can help students in their learning, and understanding and guide them on the path of success. The chemistry quiz on various chemistry articles helps students in general and competitive examinations like class examinations, NEET, JEE, IIT, etc. Start the online quiz below to test yourself.