What is Dalton’s Atomic Theory?
Dalton’s atomic theory proposed that an atom was the smallest indivisible and indestructible building block of matter. Dalton’s atomic theory was given by English physicist and chemist John Dalton in 1808. At the turn of this century, many valuable information about atoms of the periodic table elements was given. Today electrons hold the key to the chemical world−descibibe structure and chemical bonding of matter. The most important factor in physics and chemistry for the determination of the structure has been given by the study of the electromagnetic spectrum emitted or absorbed by atoms. Therefore, the modern structure investigated by electronic configuration and atomic spectrum is different from Dalton’s atomic model.
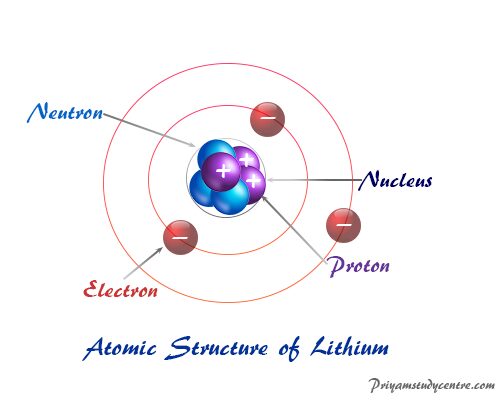
Dalton’s atomic theory provided an explanation for the law of conservation of mass and the law of definite proportions. According to Dalton’s theory, all matter (whether an element, a compound, or a mixture) is composed of small particles, called atoms.
The modern atomic structure clearly indicates an atom composed of subatomic elementary particles such as electrons, protons, and neutrons. Therefore, Dalton’s atomic theory no longer granted its position.
Postulates of Dalton’s Atomic Theory
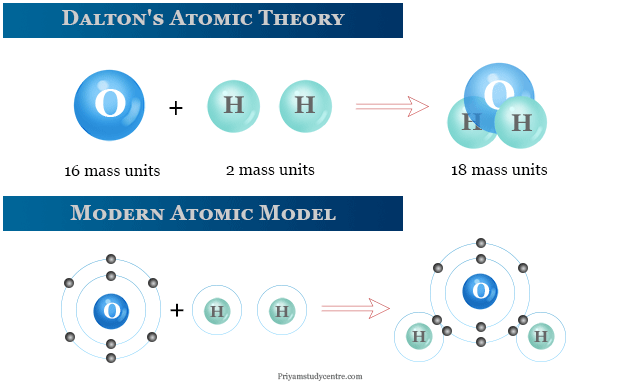
For learning chemistry or physics, Dalton’s atomic theory or model is based on the following postulates or assumptions,
- Every matter is made up of very small particles called atoms.
- Atoms are indivisible and indestructible particles that can neither be created nor destroyed in a chemical reaction.
- All the atoms of a given element have identical atomic mass and chemical properties.
- Atoms present in different elements have different masses and chemical properties.
- Chemical compounds are formed by the combination of two or more same or different kinds of atoms in a ratio of a small whole number. Therefore, the relative numbers and kinds of atoms are constant in a given compound.
- A chemical reaction is a rearrangement of atoms in matter or chemical compounds.
Merits of Dalton’s Atomic Theory
After the discovery of the modern structure of atoms, Daltons, theory no longer granted its position but it proved to be very useful in various ways:
- Dalton’s theory can explain successfully the laws of chemical combination and the law of conservation of mass.
- Dalton was the first scientist who recognize a workable distinction between the ultimate particle of an element (atom) and a compound (molecule).
Limitations of Dalton’s Atomic Theory
- Dalton’s atomic theory does not account for subatomic particles such as electrons, protons, and neutrons. It states that atoms are indivisible and indestructible parts of matter.
- It does not explain the isotopes of elements because Dalton’s postulates state that all atoms of an element have identical masses.
- Dalton’s atomic theory states that the masses of the atoms of two different elements must differ but after the discovery of isobers, it is possible for two different elements to share the same mass number. For example, 40Ar and 40Ca are two different atoms but their mass number are similar.
- It states that atoms of two or more elements combine in a ratio of a small whole number to form compounds but atoms of certain complex organic compounds such as sugar/sucrose (C11H22O11) do not combine in a ratio of a small whole number.
- Dalton’s atomic theory does not differentiate the properties of carbon allotropes such as diamond and graphite. Both of these allotopes contain only carbon atoms but they show different structure and properties. This phenomenon cannot be explained by Dalton’s atomic theory.
What are Atoms?
Atoms can be defined as the smallest particles of an element which may or may not have independent existence but take part in a chemical reaction. For example, atoms of hydrogen, nitrogen, oxygen, and chlorine are not capable of independent existence whereas atoms of helium, neon, and argon are capable of existing independently.
Atoms of most elements are not able to exist independently and they form molecules or ions. These molecules or ions aggregate in large numbers to form the substances or matter that we can see, feel, and touch. Therefore, atoms are the building blocks of all substances or matter. They are very small and their radius is measured in nanometers.
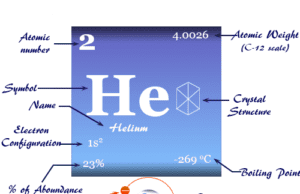
Symbols of Atoms of Elements
John Dalton Atomic Symbols
In chemistry, symbols can be defined as a short−hand representation of the name of an element. John Dalton was the first scientist who introduced atomic symbols for representing atoms of elements.
Dalton’s represented symbols for elements were difficult to draw and inconvenient to use. Therefore, a Swedish chemist, JJ Berzilius introduced the modern symbol for elements based on their Latin name.
Names and Symbols of Elements in Periodic Table
In the beginning, the names of the elements were derived from the name of the place where they were found in first time. Nowadays, IUPAC (International Union of Pure and Applied Chemistry) approves the names and symbols of the elements.
Many of the symbols can be derived from the first one or two letters of the element’s name in English. The first letter of a symbol is always written in a capital letter and the second letter is a small letter. The names and symbols for some periodic table elements based on the first one or two letters of the element’s name in English are given below the table:
| Name | Symbol | Name | Symbol |
| Hydrogen | H | Helium | He |
| Boron | B | Lithum | Li |
| Carbon | C | Berrylim | Be |
| Oxygen | O | Aluminium | Al |
| Nitrogen | N | Silicon | Si |
| Fluorine | F | Argon | Ar |
| Phosphorus | P | Scandium | Sc |
| Sulfur | S | Titanium | Ti |
| Vanadium | V | Cobalt | Co |
| Yttrium | Y | Nickel | Ni |
| Iodine | I | Zinc | Zn |
Symbols of some elements have been taken from their names in different languages such as Latin, German, Greek, etc.
- Sodium − Na from Natrium (Latin name)
- Potassium − K from Kalium (Latin name)
- Iron − Fe from Ferrum (Latin name)
- Copper − Cu from Cuprum ((Latin name)
- Silver − Ag from Argentum (Latin name)
- Tin − Sn from Stannum (Latin name)
- Antimony − Sb from Stibium (Latin name)
- Tungsten − W from Wolfram (Latin name)
- Gold − Au from Aurum (Latin name)
- Mercury − Hg from Hydrargyrum (Latin name)
- Lead − Pb from Plumbum (Latin name)
Rutherford Atomic Model
To learn more about the Rutherford Model of Atom
JJ Thomson was the first scientist who propose a model for the structure of an atom. It can be compared to a watermelon, in which, the positive charge in an atom is spread all over the red edible part. The electron particles are studded in the positively charged sphere like the seeds in a watermelon.
Science goes step by step and every scientist depends on the work of his predecessor. When we turn this century’s Rutherford model or alpha ray scattering experiment has given valuable information about the nucleus of an atom and extranuclear electrons. On the basis of his experiment, he put forward the Rutherford nuclear model of an atom, having the following features:
- All the positive charges and the almost entire mass of an atom are concentrated in a very small central core. He named this part the nucleus of an atom.
- The nucleus of an atom is surrounded by negatively charged particles carrying negligible mass, called electrons. The electrons revolve around the nucleus of an atom in a circular path.
Rutherford suggested that his model of atoms was similar to that of our solar system. In the solar system, the different planets revolve around the Sun. Similarly, in an atom, the electrons revolve around the nucleus of an atom.
Bohr’s Model of Atom
To learn more about the Bohr Model of Hydrogen Atom
The model derived by Rutheford could not explain the stability of an atom. When charged electrons are moving under the attractive force of a positively charged nucleus, they emit radiation, and the particle loses kinetic energy and hits the nucleus.
It also does not explain the distribution of electrons in the extranuclear portion of an atom and the classical model of electromagnetic radiation.
To overcome the limitations raised against Rutherford’s atomic model, Neils Bohr put forward the following postulates about the model of an atom:
- An atom consists of a positively charged nucleus around which electrons revolve in discrete orbits. Therefore, electrons, revolve in certain permissible orbits and not just any orbit.
- Each of these atomic orbits is associated with a certain value of energy. Therefore, these orbits are called energy shells or energy levels.
- An electron can jump from one orbit to another with higher energy on the absorption of energy. From one orbit to another lower energy orbit with the emission of energy.
- The angular momentum of an electron moving in an orbit is an integral multiple of h/2π.
Structure of Atom in Chemistry
Distribution of Electrons in Different Shells
The distribution of electrons in different energy levels or shells followed some rules. The maximum number of electrons present in a shell is calculated by the formula 2n2, where, n is the energy level or principal quantum number. Therefore, the maximum number of electrons in different shells can be calculated in the following ways:
- First orbit or K-shell = 2 × 12 = 2
- Second orbit or L-shell = 2 × 22 = 8
- Third orbit or M-shell = 2 × 32 = 18
- Fourth orbit or N-shell = 2 × 42 = 32
The electrons which participate in chemical bonding present in the outermost shell of an atom are called valence electrons. These electrons can explain the chemical properties of atoms.
The atoms of elements having a completely filled outermost shell which has eight electrons show little chemical activity and high stability. Such elements are called inert elements and their valency is zero. Of these inert atoms, the helium (He) atom has two electrons in its outermost shell.
Modern Structure of Atom
Bohr’s and Sommerfield’s theories could not explain the fine structure and wave properties of atoms. The particles and wave properties of electrons can be explained by Planck’s quantum theory and de Broglie relation.
Bohr and Sommerfield’s theory indicated the principal and azimuthal quantum number of atoms. These two quantum numbers are not sufficient to specify the fine structure of the atoms. Therefore, along with these two quantum numbers another two quantum numbers need to explain the fine structure of the atoms.
The quantum numbers are the identification numbers for electrons. The modern structure of atoms is based on quantum numbers and electron configurations or distributions in different orbitals. These factors explain the chemical properties and position of the elements on the modern periodic table.
The modern atomic structure classifies periodic table elements into s-block, p-block, d-block, and f-block elements on the basis of the outer orbitals in which the outer electron/electrons can stay.
Frequently Asked Questions – FAQs
How can Dalton’s atomic theory explain the law of conservation of mass?
According to Dalton’s atomic theory, when a reaction takes place, atoms of elements only rearrange but neither atoms are created nor destroyed. It suggests that the amount of matter should be constant before and after the chemical reaction.
A matter has a definite mass. Therefore, the sum of the masses before the reaction should be equal to the sum of the masses after the reaction. It successfully explains the law of conservation of mass.
For example, one water molecule is formed by the chemical reaction between two hydrogen atoms and one oxygen atom.
Similarly, 18 g of water formed by a chemical reaction between 2 g of hydrogen and 16 g of oxygen. Therefore, there is no loss or gain of mass after the reaction.
Who named the atom?
The name atom was coined first by the Pre-Socratic Greek philosopher Leucippus and his pupil Democritus in c.460–c.370 BC) It was obtained from the Greek word atomos meaning uncuttable.
What are atoms made of?
Atoms basically made of three subatomic particles − electrons, protons, and neutrons. An atom consists of a positively charged nucleus surrounded by one or more negatively charged electron particles.
Why are atoms neutral?
The atoms are electrically neutral because the number of positively charged protons and negatively charged electrons are equal. Therefore, the opposite charges are balanced, and the net charge on the atom is zero.
Can atoms be destroyed?
An atom is the smallest part of an element that bears a definite mass. The law of conservation of mass states that the mass of matter can neither be created nor destroyed during a chemical reaction. Therefore, atoms can neither be created nor destroyed during a chemical reaction.
Two or more atoms only rearrange to form a new chemical species. Dalton’s atomic theory also says that atoms can not be destroyed but they contain smaller particles such as electrons, protons, and neutrons.
How many atoms are in oxygen?
Two oxygen atoms combine covalently to form one oxygen molecule. Therefore, two oxygen atoms are in one oxygen molecule.
Are atoms indivisible?
According to John Dalton’s atomic theory, atoms are indivisible parts of an element but after the discovery of electrons protons, and neutrons, atoms are divisible.