Analytical Chemistry Instruments
Analytical chemistry is the branch of science that uses several analysis techniques or methods and analytical instruments to identify an unknown chemical substance or matter quantitatively as well as qualitatively. Identification of a substance, elucidation of its structure, and quantitive analysis of its composition are covered in modern analytical chemistry. A large number of journals use analytical techniques, methods, and instruments for inorganic, organic, biochemistry, and forensic science analysis. The most difficult task for analytical chemists is to explain the exact branch of analytical works. Generally, we can say, it is the branch of science where a large number of research workers have working for its development. For example, all chromatographic or electrophoresis techniques were developed by biochemists or biological scientists but mass spectrophotometry was discovered by physicists. They do not like to call themselves analysts or analytical chemists for various reasons.

Analytical Techniques
The techniques used in the field of analytical chemistry for the determinations of analytes or samples are generally two types such as classical technique and instrumental technique.
Classical Techniques
In the classical technique, we used to check the presence or absence of a particular component in a given analyte in the lab. For example, the Kastle-Meyer test is used to identify the presence of hemoglobin in the given analyte.
Flame Tests
Flame tests are examples of classical techniques which is used to check the presence of specific elements in an analyte. When we expose the sample to a flame, the colour of the flame is changed.
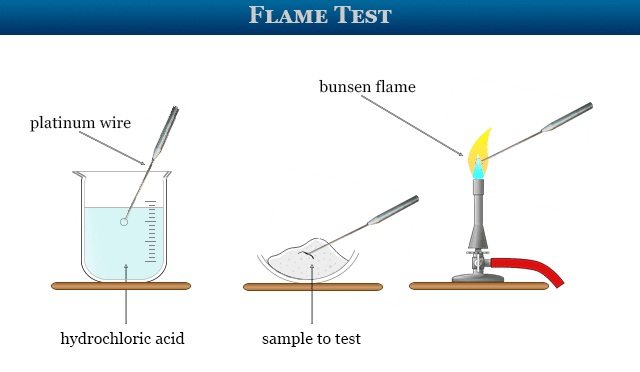
Flame Tests Experiment
- Chemically pure hydrochloric acid (HCl) is taken in a watch beaker.
- The top end of a clean platinum wire moist with HCl is held on a non-Luminus Bunsen flame.
- The process is repeated till the flame becomes colurless. The platinum wire is again moist with concentrated HCl.
- A trace of the solid sample is taken at the tip of the wire and held on the base of the non-luminous flame.
The colour change of the flame in the naked eye and the double cobalt blue glass of a particular sample is given below the table,
| Observation | Inference | |
| Colour in the naked eye | Colour through double cobalt blue glass | |
| Golden yellow | Colorless | Sodium (Na+) |
| Violet | Crimson red | Potassium (K+) |
| Brick red | Light green | Calcium (Ca+2) |
| Crimson red | Purple | Strontium (Sr+2) |
| Yellowish green | Bluish-green | Barium (Ba+2) |
| Green flame | – | Copper |
| Bluish white | – | Lead, arsenic, antimony bismuth compounds |
| Bluish-green | – | Tin compounds |
Instrumental Techniques
Different types of spectroscopic and electronic instruments are used in analytical chemistry for quantitative or qualitative analysis of samples.
- Electrophoresis or gel electrophoresis instruments are used widely in analytical chemistry for the purification or separation of proteins, amino acids, enzymes, DNA, and RNA in biochemistry.
- Spectroscopy involves the measurement and analysis of electromagnetic radiation of given atoms or molecules present in the sample.
- A common instrument that we use widely in analytical chemistry is the electrochemical cell. It is used mostly for the purification and analysis of metals.
Quantitative Analysis
In analytical chemistry, the amount of sample and range of relative amounts of constituents are important characteristics of quantitative analysis. It can be divided into three categories such as macro, semi-micro, and micro.
- In macro methods, we can analyze the sample whose weight is greater than 0.100 g.
- In semi-micro, we can analyze the sample whose weight is between 0.100 to 0.10 gm.
- In micro, we can analyze the sample whose weight is less than 0.10 g.
- A sample whose weight is less than 0.002 g is called a submicro or ultramicro sample.
The important steps for quantitative analysis are,
Sampling
It should represent the mass of the material. It can provide the martial in reasonable pure form.
Separation
The separation technique is used for the conversion of desired constituents of the sample to a measurable form. The selection of the separation technique for a specific situation depends on a number of factors. Such selection is required for accuracy and precision.
Measurement of Desired Constituent
Any physical or chemical property can be used for qualitative identification and quantitative measurement of the sample. If the property is specific and selective for measurement, then separation and pretreatment of the sample can be minimized.
Calculation and Interpretation of Analytical Data
An analysis is not complete until the results are expressed in such a manner that can be understood by all. In recent years greater attention has been given to statistical data on analytical chemistry. It can establish a new branch of science which is termed Chemometrics.
Applications of Analytical Chemistry
No other branch of science finds such extensive application as analytical chemistry. It has numerous applications in various fields of chemistry such as inorganic, organic, physical, and biochemistry.
It has also wide applications in other fields of science such as environmental science, agricultural science, biomedical or drug research, forensic science, and space research.
- In environmental science, analytical chemistry techniques are mostly used for monitoring sulfur dioxide and carbon dioxide.
- Analysis of dissolved oxygen and chlorine from water can be carried out by potentiometry and colorimetry.
- For the analysis of pesticides and insecticides from crops, we used the gas and high-performance liquid chromatography technique in analytical chemistry.
- Spectrophotometry is another instrument used for the analysis of micronutrients such as iron, copper, zinc, molybdenum, boron, and manganese in the soil sample.
- In the field of electronics, analytical chemistry is used for the analysis of traces of elements such as silicon or germanium in semiconductors and transistors.
- In the field of oceanography, earth science, planetary science, and analytical chemistry are extensively used for the analysis of seawater, blastic rocks, and lunar samples.