Oxidation Number of Periodic Table Elements
Oxidation number or state of periodic table elements in a chemical compound or molecule is the formal charge (positive or negative) which assigned to the element if all the bonds in the compounds are ionic. In chemistry, the oxidation number or state is defined as the total number of electrons lost or gained by atoms or ions to form a chemical bond.
The less or more electronegative partner of a binary compound arbitrarily assigned positive or negative oxidation numbers or states of the periodic table elements.
- Halogens (group 18) elements like fluorine (F), chlorine (Cl), and bromine (Br) are highly electronegative.
- Similarly, alkali metals or alkaline earth metals like sodium (Na), potassium (K), and calcium (Ca) are highly electropositive.
Halogens in the periodic table commonly show negative, and alkali metals or alkaline earth metals commonly show positive oxidation states or numbers.
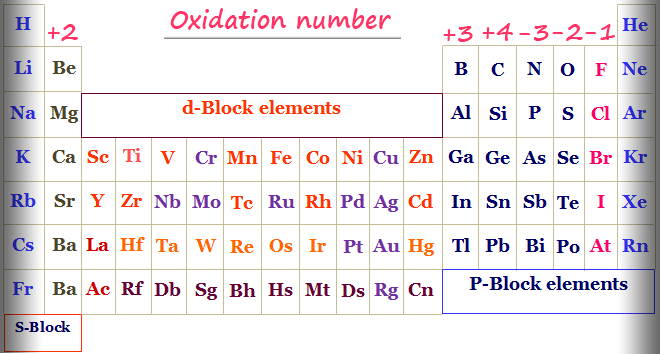
Periodic Table with Oxidation Number
Some general rules are used for the calculation of the oxidation numbers of s, p, d, and f-block elements in the periodic table.
- The s-block elements commonly show +1 and +2 oxidation numbers.
- p-block elements commonly show +3, +4, -3, -2 and -1 oxidation numbers.
- One of the most important properties that distinguish transition metals or d-block elements from non-transition elements is variable oxidation numbers or states.
- f-block elements also show variable oxidation numbers or states.
How to Find the Oxidation Number of Elements?
Some general rules used in learning chemistry to find out the oxidation number of the periodic table elements. The following general rules are used to calculate the oxidation numbers of an element in a free state or compounds,
- Rule 1: The oxidation number of atoms of the diatomic molecules like chlorine (Cl2), oxygen (O2), hydrogen (H2), and nitrogen (N2) is zero. Since the same elements of similar electronegativity are involved in the bonding of diatomic molecules.
- Rule 2: Similarly, the oxidation number of metallic elements like aluminum (Al), iron (Fe), zinc (Zn), copper (Cu), sodium (Na), and calcium (Ca) is also zero oxidation number.
- Rule 3: The common oxidation number of hydrogen = +1. In alkali metal hydrides like lithium hydride, sodium hydride, and cesium hydride, the oxidation state of the hydrogen atom = −1.
- Rule 4: All the metal in a compound generally possesses a positive oxidation state.
- Rule 5: The normal oxidation number of oxygen in a compound = −2 but in peroxides like hydrogen peroxide (H2O2) and superoxide, oxygen is assigned −1 and −½ oxidation states.
- Rule 6: The oxidation numbers of the ions in polar molecules are calculated by their charge. The algebraic sum of the oxidation numbers of all the atoms in a compound must be zero but many atomic ions equal its charge.
Why do we Calculate Oxidation Numbers?
To determine or balance common redox reactions, we used the oxidation numbers rule because some of the reactions can not be explained by electronic or classical concepts.
For example, a water molecule is formed by bonding hydrogen with oxygen, and hydrochloric acid is formed by bonding hydrogen with chlorine. The formation of water (H2O) and hydrochloric acid (HCl) molecules can not be explained from the classical definition but is explained easily by oxidation number rules.
Oxidation Numbers with Examples
| Examples of oxidation numbers in compounds | |||
| Compound | Oxidation number of elements | ||
| H2O2 | H = +1 | O = −1 | |
| CaH2 | Ca = +2 | H = −1 | |
| CHCl3 | C = +2 | H = +1 | Cl = −1 |
| Ba(MnO4)2 | Ba = +2 | Mn = +7 | O = −2 |
| K2MnO4 | K = +1 | Mn = +6 | O = −2 |
| H4P2O7 | H = +1 | P = +5 | O = −2 |
| CH2Cl2 | C = 0 | H = +1 | Cl = −1 |
Oxidation Number of Hydrogen
The electron configuration of hydrogen, 1s1. Therefore, like alkali metals hydrogen has a single electron particle in the outer quantum orbital. Therefore, hydrogen can easily lose one electron to show the oxidation number +1.
Like halogen, hydrogen is just one electron short of the next noble gas helium configuration. Therefore, it can also gain one electron from alkali or alkaline earth metals to show the −1 state.
In sodium hydride (NaH), lithium hydride (LiH), cesium hydride (CsH), and calcium hydride (CaH2), hydrogen shows an exceptional oxidation state = −1, since the common state of hydrogen = +1.
Alkali and Alkaline Earth Metals
Alkali and alkaline earth metals are highly electropositive with very low ionization energy. Therefore, alkali and alkaline earth metals always represented positive oxidation states or numbers. For example, in alkali halides, halogen shows negative oxidation states but alkali and alkaline earth metals show positive states.
The electrolysis of alkaline hydrides like lithium hydride (LiH), cesium hydride (CsH), and calcium hydride (CaH2) can liberate hydrogen gas at the anode.
Superoxide and Peroxide
Alkali and alkaline earth metals react with oxygen to form a list of binary compounds such as monoxides (M2O), peroxides (M2O2), and superoxide (MO2). These elements are the only known examples of the formation of superoxide.
The oxidation number of oxygen in alkali metals (lithium, sodium) and alkaline earth metals (magnesium, calcium) peroxide and superoxide are −1 and −½ respectively.
Fluorine is more electronegative than oxygen. It always shows an oxidation state of −1. Therefore, in F2O oxygen has oxidation number +2.
Oxidation Number Calculation
Oxidation Number of Mn in KMnO4
Let the finding oxidation number of manganese (Mn) in potassium permanganate (KMnO4) = x.
According to the above rule,
(+1) + x + 4(-2) = 0
or, x = +7
Chromium in Dichromate Ion
Let the oxidation number of chromium in dichromate ion (Cr2O7−2) = x.
Therefore, 2x + (7 × −2) = −2
or, x = +6
Both Nitrogen in Ammonium Nitrate
Ammonium nitrate (NH4NO3) is formed by a cation NH4+ and an anion NO3−. Let the oxidation number of nitrogen in NH4+ = x and NO3− = y.
For NH4+: x + 4(+1) = +1
or, x = −3
For NO3−: y + 3(−2) = −1
or, y= +5
Therefore, the oxidation numbers of both nitrogen in ammonium nitrate are −3 and +5.
Both Chlorine Atoms in Bleaching Powder
The chemical formula of bleaching powder is Ca(OCl)Cl. Hence in bleaching powder one chlorine combines with oxygen to form the OCl− ion and another chlorine atom forms the Cl− ion.
Therefore, the oxidation numbers of two chlorine atoms in bleaching powder = +1 and −1 respectively.
Phosphorus in Pyrophosphoric acid
Let the oxidation state of phosphorus in pyrophosphoric acid ( H4P2O7) = x.
∴ 4(+1) + 2x + 7(−2) = 0
or, 2x = +10
or, x = +5
Sulfur in sulfuric acid
Let the oxidation state or number of sulfur in sulfuric acid (H2SO4) = x.
∴ 2(+1) + x + 4(−2) = 0
or x = +6
Metals in Coordination Compounds
Metal ions ion in a coordination compound possess two kinds of valency−primary and secondary valency. According to the Werner theory, primary valency is equated with the oxidation state and secondary valency coordination numbers of the coordination complex.
- For example, in [Cr(NH3)6]Cl3 complex, the coordination number of chromium = 6 and oxidation number or state of chromium = +3, ammonia (NH3) molecule = 0, and chlorine ion (Cl−) = −1.
- In the iron pentacarbonyl or Fe(CO)5 complex, the oxidation state of carbonyl (CO) and iron is zero.
Oxidation Number of Carbon in Organic Compounds
Oxidation numbers of the list of hydrocarbon or carbon compounds like methane (CH4), methyl chloride (CH3Cl), dichloromethane (CH2Cl2), chloroform (CHCl3), and carbon tetrachloride (CCl4) are −4, −2, 0, +2, +4 respectively.
However, sugar, glucose, and formaldehyde are examples in organic chemistry where the oxidation number or state of the chemical element carbon on these compounds is always zero.
Carbon in Acetone Compounds
Let the oxidation numbers of carbon in acetone compounds = x and hydrogen and oxygen +1 and −2 respectively.
According to the above rule,
3x + 6(+1) + (−2) = 0
or x = −(4/3)
Oxidation number problems
Problem: How to determine the oxidation state of phosphorus in Ba(H2PO2)2?
Solution: According to the rules, the oxidation state hydrogen and oxygen in Ba(H2PO2)2 are +1 and −2 respectively, and phosphorus = x.
∴ (+2) + 2{2(+1) + x + 2(−2)} = 0
or, x = +1
Problem: Calculate the oxidation state of iron in [Fe(H2O)5(NO)+]SO4.
Solution: Let the oxidation number of iron in [Fe(H2O)5NO+]SO4 = x. The oxidation numbers of water, NO+, and sulfate ions are 0, +1, and −2 respectively.
∴ x + 5(0) + (+1) − 2 = 0
or, x = +1
Problem: How to find the oxidation state of chromium in CrO5 in chemistry?
Solution: Due to the peroxy linkage oxidation number or state of chromium in CrO5 = +6.