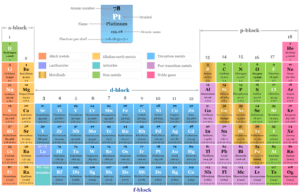
Atomic Number of Elements
Atomic number of chemical elements in chemistry is the number of protons of an atom by which the elements are arranged in the periodic table. The modern periodic system is formed on the basis of atomic number and electronic configuration but Mendeleev’s classification is based on the atomic weight or mass of an element.
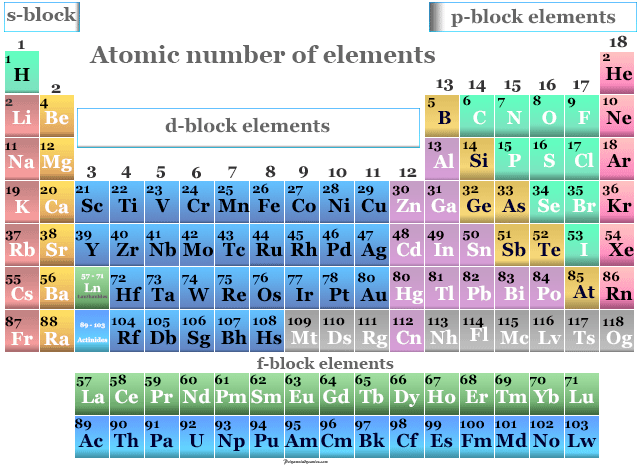
In learning chemistry, a good correlation between electromagnetic spectrum (X-ray) and atomic number indicated that an element is characterized by its atomic number, not atomic weight. The trends and properties like ionization energy, electron affinity, and shielding effect of an atom are better described by its atomic number.
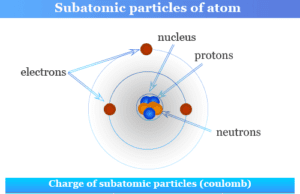
The atomic number is denoted by Z and written as a superscript towards the left of the symbol. For Example, 2He4 and 3Li7, Z = 2 and 3 for helium (He) and lithium (Li) respectively. In a neutral atom, atomic number = number of protons = number of electrons.
Moseley Atomic Number experiment
Rutherford model or alpha ray scattering experiments allow for the determination of the positive charge of the nucleus of an atom. The identification of the nuclear charge and the atomic number of an element was established by electromagnetic spectrum data found from the x-ray discovered by Moseley.
- When a beam of high-energy electrons falls on the metal target, the atom of the metal is excited and emits electromagnetic radiation in the form of energy.
- Moseley experimented with over thirty metals from aluminum to gold.
- Mosely plotted the square root of wavelength for each element against atomic weight.
- A straight line plot was obtained but few elements are out of line.
- A better plot was obtained when atomic weight was replaced by the order number or the serial number of the elements in the periodic table.
The most reasonable interpretation of the Mosely experiment is that the atomic number of elements equals the number of protons in the atomic nucleus. To maintain electroneutrality, the atom must carry an equal number of extranuclear electrons.
Mass Number of Elements
The mass number or atomic mass number (symbol A) is the total number of protons and neutrons in an atomic nucleus. Protons and neutrons together are called nucleons. Therefore, the mass number is also called the nucleon number. The mass number is denoted by the symbol A and is the nearest atomic weight integer.
If an element has mass number A and atomic number Z. The shortfall of (A − Z) determines the number of neutrons in the nucleus of an atom.
For example, the atomic number and mass number of hydrogen are equal to one. Therefore, the hydrogen atom does not carry any neutrons inside the nucleus.
The atomic number of boron = 5 and mass number = 11 Therefore, the number of neutrons
= (11 − 5)
= 6
Atomic Mass of Elements
The mass of an individual atom is called atomic mass or atomic weight. It is nearly equal to the mass number and nearly twice the atomic number for many atoms.
For example, atomic number (Z) of carbon = 6,
Mass number (A) = 12,
Atomic mass = 12.01
Atomic number (Z) of oxygen = 8,
Mass number(A) = 16,
Atomic mass = 15.99
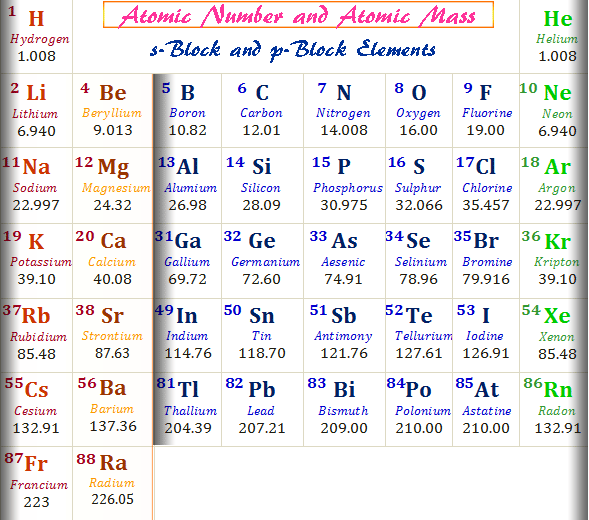
According to Dalton’s atomic theory, each element has a characteristic atomic mass. The determination of the mass of an individual atom was a difficult task due to its very small size.
Oxygen Scale of Atomic Mass
The relative atomic masses can be determined by using the law of chemical combinations. In the oxygen scale, 1/16 of the mass of an atom of naturally occurring oxygen was taken for the determination of atomic weight. It is taken standard due to the following two reasons:
- Oxygen can react with a large number of elements to form chemical compounds.
- The masses obtained for most elements by oxygen scale is a whole number.
On the physical scale of atomic mass or weight, the natural oxygen atom (relative weight 16) is taken as the reference. But natural oxygen was found to be the mixture of three isotopes of mass number 16, 17, and 18 with the percentage of abundance 993575, 0.039, and 0.204 respectively.
Therefore, the average atomic mass of natural oxygen,
= 16 × 0.99757 + 17 × 0.00039 + 18 × 0.00204
= 16.00447
When the exact number 16.0047 is chosen as the relative mass of oxygen, the new scale of atomic weights is obtained. In 1961, carbon (C−12) was chosen as a stranded reference for calculating atomic masses universally.
Carbon−12 Scale of Atomic Mass
In the carbon−12 scale, the atomic mass of an element is defined as the number of times a given atom is heavier than 1/12th of the mass of 1 atom of carbon−12 (C−12). In another way, it is the average mass of the atom as compared to 1/12th of the mass of one carbon−12 atom.
Simply, we can say the atomic mass of an element is a number that says how heavy an atom of chemical elements is compared to one-twelfth of the carbon−12 isotope. The gram atomic weight of an element determines the atomic mass in grams.
The relative atomic weight or mass of an element is a unitless quantity but the gram atomic weight is calculated in the gram unit.
Average Atomic Mass
If the element has no isotopes, the mass of its atom is equal to the sum of protons and neutron present in it. But if an element occurs in isotopic form, then average mass can be calculated from the percentage of each isotopic form.
Average atomic mass = [(atomic mass of isotope I × percentage of isotope I) + (atomic mass of isotope II × percentage of isotope II)…]
For example, the two isotopic forms of two chlorine atoms with masses 35 u and 37 u occur in a ratio of 3:1. Therefore, we calculate the average atomic mass of a chlorine atom by the above formula.
The average atomic mass of a chlorine atom
= (35 × 0.75) + (37 × 0.25)
= 35.5u
Here, 35.5 u is not the atomic mass of any individual chlorine atom but it is the average atomic mass of chlorine calculated from the percentage of each isotopic form.
Atomic Mass Unit
The mass of a single atom in grams is too small a number. Therefore, we express it by atomic mass unit (amu). One atomic mass unit is exactly equal to 1/12th of the mass of the one atom of carbon−12 isotope.
Earlier, the atomic mass unit was represented by the symbol amu but according to the latest IUPAC recommendation, it is written as “u”−unified mass.
One amu (u) is numerically equal to the mass of one atom of carbon on a carbon−12 isotope.
Therefore, 1 amu = 1.660054 × 10−24 g
= 1.66054 × 10−27 kg
The atomic mass of the first thirty periodic table chemical elements is given below in the table,
| Atomic number | Name | Symbol | Atomic mass (amu or u) |
| 1 | Hydrogen | H | 1.008 |
| 2 | Helium | He | 4.026 |
| 3 | Lithum | Li | 6.94 |
| 4 | Beryllium | Be | 9.0122 |
| 5 | Boron | B | 10.81 |
| 6 | Carbon | C | 12.011 |
| 7 | Nitrogen | N | 14.007 |
| 8 | Oxygen | O | 15.999 |
| 9 | Fluorine | F | 18.998 |
| 10 | Neon | Ne | 20.180 |
| 11 | Sodium | Na | 22.990 |
| 12 | Magnesium | Mg | 24.305 |
| 13 | Aluminium | Al | 26.982 |
| 14 | Silicon | Si | 28.085 |
| 15 | Phosphorus | P | 30.974 |
| 16 | Sulfur | S | 32.06 |
| 17 | Chlorine | Cl | 35.45 |
| 18 | Argon | Ar | 39.948 |
| 19 | Potassium | K | 39.098 |
| 20 | Calcium | Ca | 40.078 |
| 21 | Scandium | Sc | 44.956 |
| 22 | Titanium | Ti | 47.867 |
| 23 | Vanadium | V | 50.942 |
| 24 | Chromium | Cr | 51.996 |
| 25 | Manganese | Mn | 54.938 |
| 26 | Iron | Fe | 55.845 |
| 27 | Cobalt | Co | 58.933 |
| 28 | Nickel | Ni | 58.693 |
| 29 | Copper | Cu | 63.546 |
| 30 | Zinc | Zn | 65.38 |
Molecular Mass
Atoms of most chemical elements are not able to exist independently and form molecules or ions. These molecules or ions aggregate in large numbers to form the matter that we can see, feel, and touch.
The molecular mass of a substance is the sum of the atomic masses of all the atoms present in a molecule of the substance. Therefore, the relative mass of a molecule is expressed in atomic mass units (amu or u). For example, the relative molecular mass of water (H2O) is 18 u and calculated in the following way,
A water (H2O) molecule is formed by combining two hydrogen atoms and one oxygen atom.
The atomic mass of hydrogen = 1 u
The atomic mass of oxygen = 16 u
Therefore, the molecular mass of water (H2O)
= 2×1 + 1×16
=18 u
When molecular mass is expressed in grams is called gram molecular weight.
Molecular Mass Calculation
How to calculate the molecular mass of the following substances where the atomic mass of H = 1, N = 14, Cl = 35.5, O = 16, P = 31 u, and S = 32 u?
- Ammonia (NH3)
- Hydrochloric acid (HCl)
- Sulfuric acid (H2SO4)
- Nitric acid (HNO3)
- Phosphorus (P4) molecule
- Hydrogen sulfide (H2S)
- Hydrogen peroxide (H2O2)
- Sulfur dioxide (SO2)
We calculate the molecular mass of a substance by adding the atomic masses of all the atoms present in a molecule.
- The molecular mass of ammonia (NH3)
= 1 × 14 + 3 × 14
= 17 u - The molecular mass of hydrochloric acid (HCl)
= 1 × 1 + 1 × 35.5
= 36.5 - The molecular mass of sulfuric acid (H2SO4)
= 2 × 1 + 1 ×32 + 4 × 16
= 98 u - The molecular mass of nitric acid (HNO3)
= 1 × 1 + 1 × 14 + 3 × 16
= 63 u - The molecular mass of the phosphorus (P4) molecule
= 4 × 31
= 124 u - The molecular mass of hydrogen sulfide (H2S)
= 2 × 1 + 1 × 32
= 34 u - The molecular mass of hydrogen peroxide (H2O2)
= 2 × 1 + 2 × 16
= 34 u - The molecular mass of sulfur dioxide (SO2)
= 1 × 32 + 2 × 16
= 64 u
Formula Unit Mass
The ionic compounds do not consist of molecules and the term molecular mass is not correct for them. Therefore, we use the term formula mass for ionic compounds in which individual molecules do not exist.
The formula mass unit of an ionic compound is the sum of the atomic masses of all atoms present in a formula unit. We can calculate the formula unit mass in the same manner as we calculate the molecular mass.
For example, the formula unit mass of sodium chloride (NaCl)
= 1 × 23 + 1 × 35.5
= 58.5 u
The formula unit mass of potassium chloride (KCl)
= 1 × 39.1 + 1 × 35.5
= 74.6 u
Molar Mass
The mass of one mole of a chemical substance is equal to its relative atomic or molecular mass in grams. The numerical value of molar mass is similar to that of molecular mass but changes the unit from amu or u to gram. The molar mass of atoms is also called gram atomic mass.
For calculating the gram molecular mass or molar mass we keep the numerical value the same but change the units from u to g.
We know the molecular mass of water (H2O) = 18 u
Therefore, 18 u water = one molecule water
Again, 18 g water = one mole of water
= 6.022 × 1023 molecules of water
Problem: Find the molar mass of 0.2 moles of oxygen atoms and 2 moles of water molecules.
Solution: Mass of 1 mole of oxygen atoms = 16 g
Therefore, the mass of 0.2 mole of oxygen atoms
= 0.2 × 16
=3.2 g
Mass of one mole of water (H2O) molecules
= (2 × 1 + 1 × 16)
= 18 g
Therefore, the mass of 2 moles of water molecules
= 2 × 18
= 36 g
How to Find the Atomic Weight of an Element?
Scientists Stas in chemistry use silver and chlorine to find the atomic weight or mass of the chemical elements.
- He has taken the known weight of pure silver heated with pure chlorine in the glass tube.
- After the reaction the resulting silver chloride weighed. He also dissolved a known quantity of pure silver crystal in pure nitric acid precipitated the silver as AgCl and weighed it.
- He found that 35.45 g of chlorine combines with 107.94 g of silver to give 143.39 g of AgCl.
- Therefore, the calculated atomic weight or mass of chlorine is equal to 35.45 and silver = 107.94.
Frequently Asked Questions – FAQs
What atomic number is gold?
Gold (Au) is a group 11 metal with atomic number 79 and an atomic mass of 196.97.
How is atomic mass determined?
Atomic mass can determined by adding masses of the protons, neutrons, and electrons in an atom or calculating the average mass in a group of atoms. However, the mass of electrons is so much less than that of protons and neutrons. Therefore, they don’t factor into the determination of atomic masses.
Who arranged elements by atomic number?
In 1869, Russian chemist Dmitri Mendeleev provided the framework of the modern periodic table. He arranged the periodic table elements according to their atomic weights. In 1913, English physicist Henry Moseley rearranged such elements in the periodic table on the basis of atomic numbers.
What does the atomic number indicate?
Atmic number indicates the number of protons in the nucleus and it also indicates the number of electrons in the neutral atom. For cationic or anionic species, the number of electrons is less or greater than that of protons.
Are atomic mass and mass number the same?
They are different because atomic mass is the average mass of an atom but the mass number is the total number of nucleons (protons and neutrons) in the atomic nucleus.
Why an atomic mass unit is needed?
The mass of a single atom in grams or kg is too small a number. Therefore, we express the mass of a single atom by atomic mass unit (amu or u). Hence, we used atomic mass unit (AMU), in physics and chemistry for expressing masses of atoms.
Why atomic number is called the fingerprint of elements?
The atomic number is unique for each element and we arrange the elements in the modern periodic table based on atomic number. It is the number of protons in the atomic nucleus or the number of electrons present in a neutral atom.
Therefore, atomic number provides the periodic position of an element which gives the idea about various physical and chemical properties of an element. That is why the atomic number is the fingerprint of elements.