Nuclear Fission
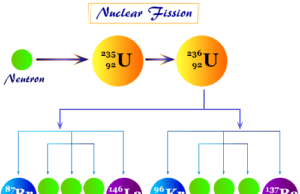
Definition of Nuclear Fission
Nuclear fission is a nuclear reaction where a heavy nucleus of an atom is split spontaneously to produce two or more smaller...
Nuclear Fusion
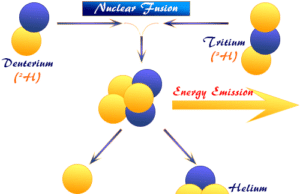
Nuclear Fusion Energy
Nuclear fusion is a nuclear reaction where very light nuclei of chemical elements can be joined or fused to form heavier elements...
Nuclear Binding Energy
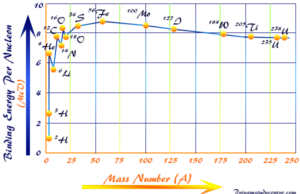
Mass Defect and Nuclear Binding Energy
Nuclear Binding energy and mass defect can be calculated from the expected mass of any nucleus from the knowledge...
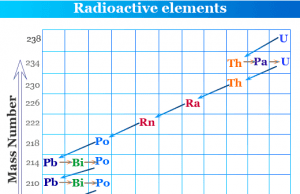
Radioactive Isotopes
What are Radioactive Isotopes?
Radioactive isotopes or radioisotopes of a radioactive element are atoms of the same element with different mass numbers. Isotopes of a...
Alpha Beta Gamma
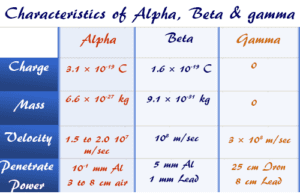
Alpha Beta Gamma Rays Properties
Alpha, beta, gamma rays radiation in radioactivity is the spontaneous radioactive decay of atomic particles in the form of energy...
Radioactivity
Measurement of Radioactivity
Radioactivity is the phenomenon of emission of radiations as a result of the spontaneous decay of alpha, beta, and gamma particles or...
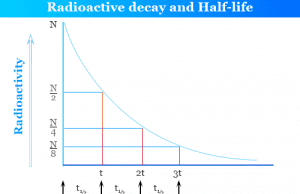
Radioactive Decay
Radioactive Decay and Half Life
Radioactive decay is the spontaneous disintegration or emission of atomic particles like alpha, beta, gamma from the nuclei of radioactive...