Structure of Molecule
A molecule in chemistry or biology is the smallest identifiable structure formed by two or more atoms and it retains the chemical properties of formation substances. Different types of forces or chemical bonding are responsible for the formation of most types of molecules. According to the polarity and structure of molecules, they are of two types, polar (water, hydrogen chloride, ammonia, etc) and nonpolar (carbon dioxide, benzene, boron trifluoride, etc) molecule. In many crystalline solid molecules, the atoms are the structural units and these atoms are held together by ionic, covalent, or hydrogen bonding. For example, in learning chemistry, the crystalline form of organic compounds like alcohol and carboxylic acid is supported by hydrogen bonding.
Sodium chloride (NaCl) and potassium chloride (KCl) are well-known examples of molecules formed by ionic bonding. However, zinc sulfide, silicon carbide, silver iodide, and water are examples of molecules formed by covalent bonding.
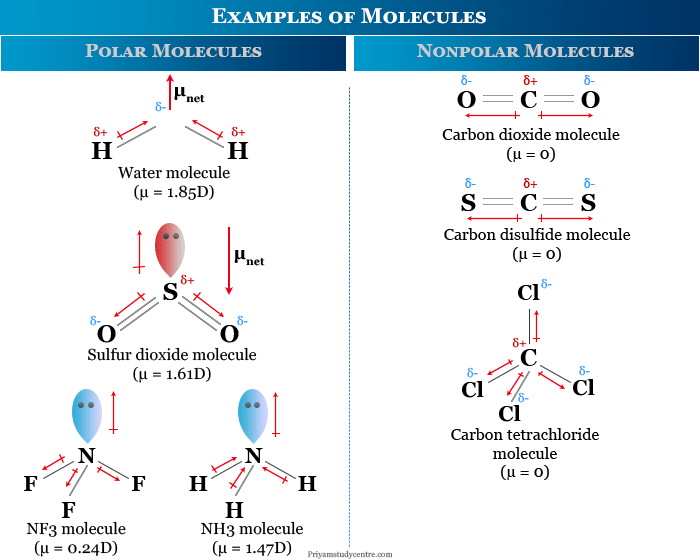
Examples of Polar and Nonpolar Molecules
Covalent molecules are composed of positively charged nuclei and negatively charged electrons distributed in space. According to the structural arrangement and polarity, molecules are two types
- Polar molecules
- Nonpolar molecules
Polar Molecule
A polar molecule in chemistry is one in which there is a separation of the centers of gravity of positive and negative charges. The molecule developed a positive and a negative pole. Normally, heteronuclear diatomic molecules are polar due to the difference in electronegativity in bonding atoms.
The polar character of a molecule is mostly described by the term dipole moment. Examples of some common polar molecules are,
| Example of molecule | Molecular formula | Dipole Moment (Debye) |
| Hydrogen fluoride | HF | 1.91 |
| Water | H2O | 1.85 |
| Hydrogen sulfide | H2S | 1.10 |
| Hydrogen chloride | HCl | 1.03 |
| Hydrogen bromide | HBr | 0.78 |
| Ammonia | NH3 | 1.46 |
| Sulfur dioxide | SO2 | 1.63 |
| Carbon monoxide | CO | 0.12 |
| Nitrous oxide | N2O | 0.17 |
| Nitrogen trifluoride | NF3 | 0.20 |
Nonpolar Molecule
The nonpolar molecule in chemistry is one where the center of gravity of the positive charge due to nuclei coincides with the center of gravity of the negative charge due to electron.
Binary homonuclear molecules are nonpolar since the bonding elements possess the same electronegativity. Examples of some common nonpolar molecules are,
| Examples of molecules | Molecular formula | Dipole Moment (Debye) |
| Hydrogen | H2 | 0 |
| Chlorine | Cl2 | 0 |
| Phosphorus pentachloride | PCl5 | 0 |
| Carbon dioxide | CO2 | 0 |
| Carbon disulfide | CS2 | 0 |
| Carbon tetrachloride | CCl4 | 0 |
| Boron trifluoride | BF3 | 0 |
| Benzene | C6H6 | 0 |
| Stannic chloride | SnCl4 | 0 |
| Beryllium chloride | BeCl2 | 0 |
Atoms and Molecules
Mostly the atoms are held together through ionic, covalent, and metallic bonding to form the three-dimensional structure of molecules.
- The ionic molecules generally formed a three-dimensional crystal structure where atoms or groups of atoms occupied crystal positions.
- Covalent molecules are formed by sharing electron pairs between atoms.
- In the metallic crystal, multiple atoms are arranged in a regular pattern through metallic bonding.
After the development of quantum mechanics, two alternative theories and hybridization are used to explain the structure of different types of covalent molecules. These theories are valence bonds and molecular orbital theory.
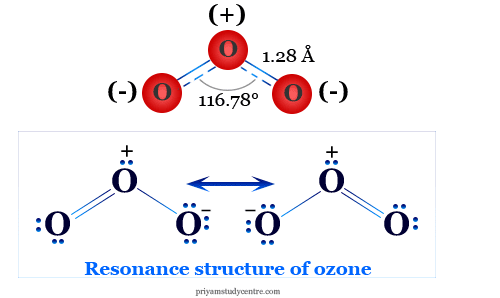
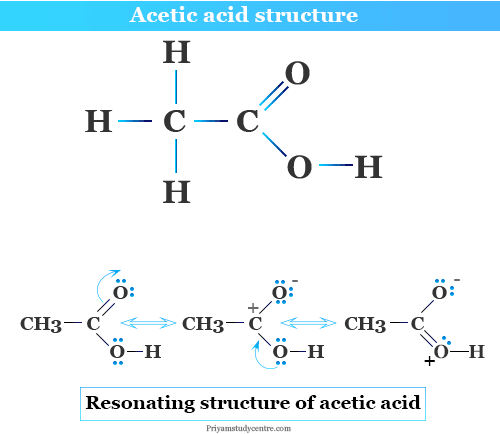
Resonance Structure in Chemistry
When the chemical properties of the molecules cannot be described by a single structural diagram, they form different types of molecular structures, the true structure in chemical science means the resonance hybrid of the pure molecule. Resonance provides the different types of electronic structure of molecules.
The resonance structure of acetic acid and ozone gas molecules is given below the picture,
Hybridization in Chemistry
In chemistry, hybridization is the mixing of pure atomic orbitals to give an equal number of hybridized orbitals. For example, the carbon atom in the methane molecule is sp3 hybridized.
Hybridization Rules
- Only orbitals of similar energies belonging to the same atom or ion can be hybridized together.
- The number of hybridized orbitals produced is equal to the number of orbitals undergoing hybridization.
- Most of the orbitals are similar but they are not necessarily identical shapes.
- From the type of hybridization, one can also predict the geometry and bond angle of a molecule in chemistry.
Types of Hybridization
sp, sp2, and sp3 are the most common types of hybridization for the molecules. The type of hybridization predicts the shape of the molecules. Some common examples are given below the table,
| Molecule | Types of hybridization | Shape of Molecules |
| BeCl2 | sp | linear |
| CO2 | sp | linear |
| BCl3 | sp2 | trigonal planar |
| SnCl2 | sp2 | V-shape |
| CH4 | sp3 | tetrahedral |
| H2O | sp3 | V-shape |
| PF5 | sp3d | trigonal bipyramidal |
| ClF3 | sp3d | T-shape |
| SF6 | sp3d2 | octahedral |
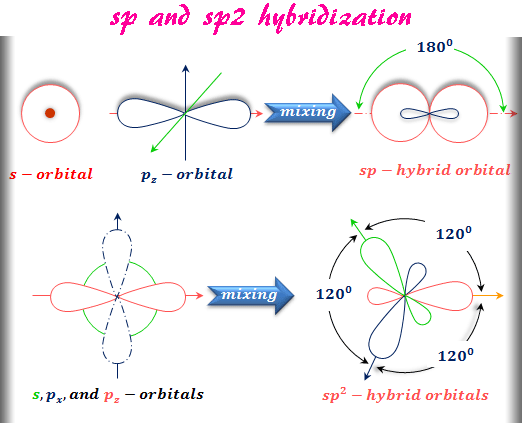
sp Hybridization
A linear combination of one s and one px orbitals in suitable proportion gives two sp hybridized orbitals. This type of hybridization is called sp hybridization. The mixing of one s and one px is given below the picture,
sp2 Hybridization
The mixing of one s and two p (px and py) atomic orbitals gives three equivalent sp2 hybridized orbitals. The formation of sp2 hybridized orbitals is given above the picture. In sp2 hybridization, the hybridized orbitals formed a trigonal planner structure.
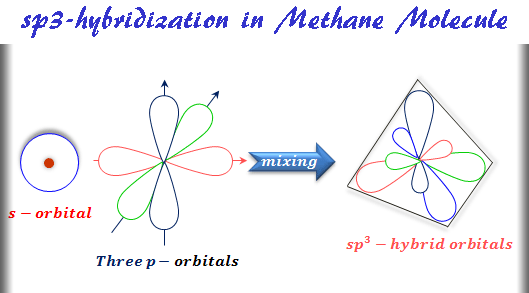
sp3 Hybridization
When one s and three p atomic orbitals mix, they form four equivalent sp3 hybridized orbitals. Such a type of mixing of atomic orbitals is called sp3 hybridization. The formation of tetrahedral sp3 hybridized orbitals is given below the picture,
Examples of Molecules
Structure of Carbon Dioxide Molecule
A carbon dioxide molecule is an example, formed by components like one carbon and two oxygen atoms having linear structural formulas.
The valence shell electronic configuration of chemical element carbon in excited state 2s1 2px1 2py1 2pz1. Oxygen atoms (normal state) 2s2 2px1 2py1 2pz1.
The liner shape of O=C=O suggests that the carbon atom is the central atom with sp-hydridized. Two sp-hybrid orbitals of carbon atoms overlap with two oxygen atoms during the formation of two sigma bonds. Another two half-filled 2p-orbitals carbon atoms bound by pi bonds with two oxygen atoms.
The experimental bond length between carbon-oxygen in carbon dioxide molecule = 1.15 Å. The resonating structure of the carbon dioxide molecule is equivalent to a resonance bond energy of 33 kcal/mole.
Structure of BCl3
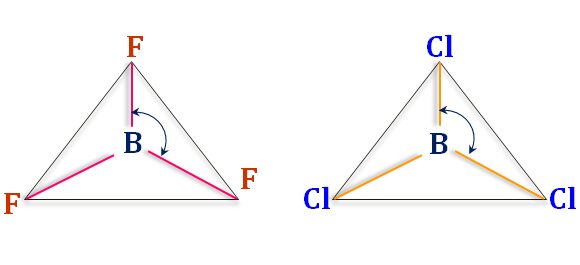 The boron atom is the central atom in the BCl3 molecule in chemistry. The valence shell electronic configuration of the boron atom in the excited state, 2s1 2px1 2pz1.
The boron atom is the central atom in the BCl3 molecule in chemistry. The valence shell electronic configuration of the boron atom in the excited state, 2s1 2px1 2pz1.
During hybridization, these atomic orbitals form three sp2 hybrid orbitals. When these hybridized orbitals overlap with three singly filled 3pz orbitals of the chlorine atom, they form three B-Cl sigma bonds. Therefore, the BCl3 molecule is a trigonal planner shape.
In this molecule central atom boron with an incomplete octet. According to the definition of Lewis acids bases, BCl3 molecule affinity to accept electron pair acts as a Lewis acid by forming a coordinate covalent bond with other molecules or Lewis bases.
Methane Molecule Structure
Methane is the simplest hydrocarbon of an alkane or paraffin molecule having molecular formula CH4. In methane molecules, the exited carbon atom has 2s1 2px1 2py1 2pz1 electronic configuration.
When mixing these four atomic orbitals, they give four equivalent sp3-hybrid orbitals. These hybrid orbitals are directed toward the corners of the regular tetrahedron. Therefore, each sp3-hybrid orbitals overlap with the 1s-atomic orbitals of four hydrogen atom by the formation of four sigma bonds in the methane molecule.
Structure of Ammonia Molecule
In the ammonia molecule nitrogen atom is the central atom, define valence shell electronic structure, 2s2 2px1 2py1 2pz1. Therefore, four atomic orbitals of nitrogen hybridized to form four equivalent sp3-hybrid orbitals.
Three of these four orbitals in ammonia molecule overlap with 1s-orbitals of hydrogen to form three sigma-bonds while the remaining hybrid orbitals contain a lone pair of electrons. Therefore, the ammonia molecule looks like a distorted tetrahedral shape with a bond angle of 107.5º.
Structure of Water Molecule
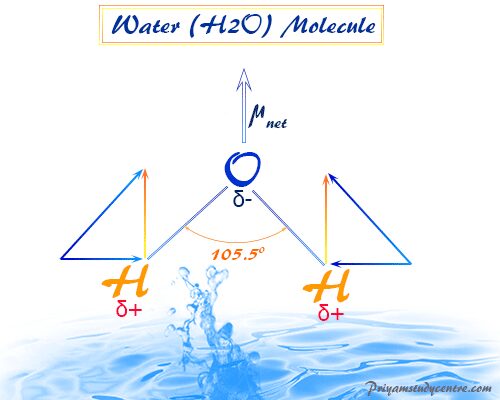
The molecular formula of the water molecule is H2O. Water molecule is formed by sp3 hybridization. Water molecules contain two bond pairs and two lone pairs.
Due to the presence of lone pairs on the central oxygen atom, the shape of the water molecule is not regular tetrahedral. Therefore, The water is a V-shaped molecule with an H-O-H bond angle of 105.5°.
Hydrogen Fluoride Molecule
The molecular formula of hydrogen fluoride is HF. Hydrogen fluoride molecule is formed by sp3-hybridization. The fluorine atom is the central atom in the HF molecule. Hydrogen fluoride molecules generally contain one bond pair and two lone pairs with linear structure.