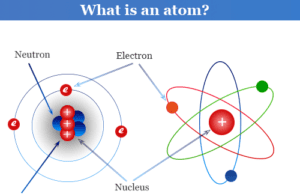
Chemistry Test Online or Quiz Questions with Answers
Chemistry online test or MCQ-2 contains 10 chemistry quiz questions answer for high school and college grade students which help for the preparation of competitive examinations like NEET, KVPY, etc. Therefore, start the below chemistry test questions to evaluate yourself.