Acids and Bases Multiple Choice Questions and Answers
Acids and bases questions or multiple choice questions (MCQs) of acids and bases contain 10 quiz questions and answers for 9, 10, 11, and 12 grade or class chemistry students. Such type of quiz set about acids and bases properties and theory can help the students in class and competitive examinations like NEET, JEE, and many other examinations. In an aqueous solution nitric and sulfuric acid completely dissociates and is almost equally strong. But when these acids are taken in glacial acid medium nitric acid is much stronger than the other two acids. Hence solvent plays an important role in explaining acid-base redox reactions. Boric acid is a weak acid but when boric acid is mixed with sulfuric acid behaves as a strong acid with strong oxidizing properties. Before participating in the quiz on acids and bases, study carefully the acids and bases definition, theory, and properties.

Start the below learning chemistry quiz or acids and bases multiple choice questions (MCQs) and answers to evaluate yourself online,
What are the Acids and Bases?
Before participating above quiz or seeing acids and bases multiple choice questions (MCQs) and answers, study carefully the acids and bases definition, theory, and properties mentioned below topics,
- Arrhenius theory
- Lewis acid base theory
- Conjugate acid base pair
- Hard soft acid base theory
- pH scale for acids and bases
We can also provide the definition of acids and bases on the below topics,
Arrhenius Theory
Our general ideas regarding acids and bases are based on the Arrhenius theory. According to Arrhenius,
- An acid is a compound or molecule that yields H+ ions in a water solution.
- A base is a compound molecule that yields OH– in water solution and utilizes readily from solvent or water.
Arrhenius’s theory explains the acid and base neutralization reaction in water solution only but it does not explain the properties in gas or solid-phase acid-base reaction.
Hence Arrhenius’s theory is very useful for explaining acid and bases in water solution but can’t explain the strength of acid in glacial acetic acid or ammonia solution.
Acidic Strength of Oxyacids of Phosphorus
The oxidation number of the phosphorus atom in the oxoacids of phosphorus, hypophosphorous, phosphorus, and phosphoric acid = +1, +3, and +5 respectively. With the increasing oxidation number acidic character also increases. Therefore, the acidic character of these acids should be in the order,
H3PO4 < H3PO3 < H3PO2
But experimental observation is the reverse order
H3PO2 ≥ H3PO3〉H3PO4. The experimental order is explained when we consider the structures of these phosphorus acids.
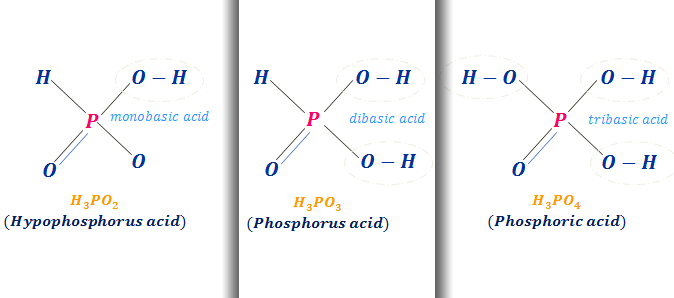
- In the H3PO2 structure, one hydrogen atom attaches phosphorus through oxygen, and another hydrogen bonding directly with phosphorus. Therefore, the proton attached to oxygen has a far greater chance of dissociation than any direct chemical bond. Hence H3PO2 dissociates one proton in the solution.
- In a similar way, H3PO3 and H3PO4 dissociate two and three protons respectively. This series contains a number of unprotonated oxygen, which is responsible for the enhancement of acidity.
Dissociable protons increase from H3PO2 to H3PO4. Therefore, the overall inductive effect of the unprotonated oxygen decreases from H3PO2 to H3PO4. Hence the acidity slightly falls off in the order, H3PO2 ≥ H3PO3〉H3PO4.
Question: Why all alkalis are bases but all bases are not alkalis?
Answer: Alkalis are the substances of our environment dissolved in water to produce bases but all the bases are not dissolved in water. Hence all the alkalis are bases but all the bases are not alkalis.
- For example, Na2O is an alkali because it is dissolved in water to produce NaOH.
- But aluminum, iron, and zinc hydroxides like Al(OH)3, Fe(OH)3, and Zn(OH)2 do not dissolve in water but react with acids to produce salt and water. Therefore, these are bases but not alkalis.
What is a Conjugate Acid Base Pair?
In 1923, Bronsted and Lowry introduced the new definition of acids and bases. According to these new concepts,
- An acid is defined as a substance that has the tendency to lose a proton.
- A base is a substance that has the tendency to gain a proton. The free proton is not likely to occur in the solution and combine with the solvent molecule.
For example, in water, glacial acetic acid, ammonia, and ethyl alcohol solvents, the proton combines to form H3O+, CH3COOH2+, NH4+, and CH3CH2OH2+ respectively.
The beauty of Bronsted Lowry’s concepts reflects the fact that they contain all effects of Arrhenius’s concepts and are further extended to explain acid-base chemical equilibrium in non-aqueous media.
Lewis’s theory of acids and bases
A more here general theory for acids was proposed on the basis of the electronic configuration of valence orbital.
According to Lewis, an acid is a substance (molecule, ion, or radical) that readily accepts an electron pair from the donor substances, which would be the base. Acid is thus an electron pair acceptor and the base is an electron pair dinner.
For example, Et3N + BCl3 → Et2N : BCl3
It is an acid-base neutralization reaction that can be followed with an indicator in chloro-benzene solution. There are no protons or OH groups involved.
The neutralization reaction means the formation of a coordinate linkage to form an adduct complex. Hard soft acid-base or HSAB-principle is also based on Lewis’s theory. This theory explains the stability of the complex in terms of the electric polarization of soft and hard acids and bases.
Acidity Trend Periodic Table
Acidic oxides react with water to give respective oxoacids. With the increasing oxidation number, electronegativity, electron affinity, and acidity increase but shielding electron of the central atom decreases the acidity of the substances. Therefore, the central chemical element plays an important role in the acidity of the substances.
Generally, electronegativity and ionization energy increase left to right in the period of the periodic table with the increasing atomic number. Hence the acidic character generally increases left to right in the periodic table.
But electronegativity decreases from top to bottom in the group of the periodic table. Hence the acidic character generally decreases from top to bottom in the periodic table.
Among Na2O, MgO, As2O3, and N2O5, nitrogen has the highest oxidation number and electronegativity. Therefore, the order of the acidic nature of these oxides is Na2O < MgO < As2O3 < N2O5.
pH Scale in Chemistry
In acid solutions, the concentration of hydrogen ions is high, and in alkaline solutions low. Sorensen suggested a new way of expressing the concentration of hydrogen ions in logarithm form.
The negative of the logarithm of H+ ion concentration or activity is defined as the pH scale.
pH = −log CH+.
For the solution pH = 3
CH+ = 10−3
For a solution pH = 8
CH+ = 10−8.
The neutral solution has a pH of 7. Any polar solution having a pH less than seven will be acid and the solution having a pH above seven will be alkaline. After studying the above topics you can easily answer the quiz on acids and bases.