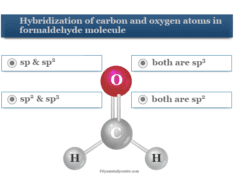
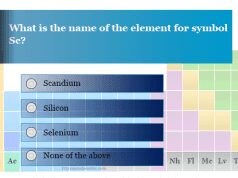
Chemistry Quiz 3 on Oxidation Number
Chemistry Quiz 3 on oxidation number of periodic table elements or redox reactions contains 10 multiple choice questions (MCQ) and answers to help for the preparation of competitive exams like NEET, IIT, JEE, etc. The oxidation number or oxidation state is a basic property of an atom that describes the total number of electrons that either gain or lose by an atom in order to form a chemical bond with another atom.
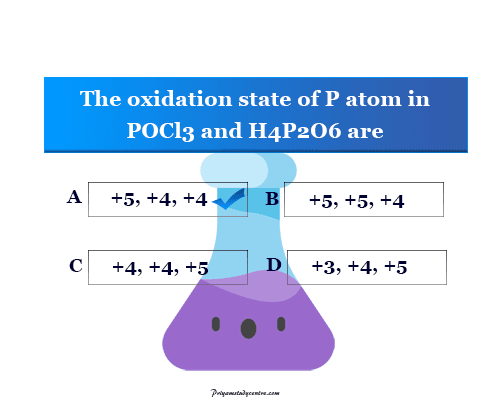
Oxidation and reduction always go hand in hand during a redox process. We can easily identify oxidation and reduction reactions with the help of oxidation numbers or oxidation states.
Such an online quiz set in learning chemistry is helping to improve the basic knowledge of oxidation reduction or redox reactions. Start the below quiz on oxidation numbers to evaluate yourself.