How does Nuclear Reaction Work?
Nuclear reaction in physics and nuclear chemistry is the type of natural and artificial transmutation of nuclei of heavy elements accompanied by the emission or splitting of particles like alpha, beta, gamma in the form of energy or electromagnetic radiation. Nuclear scientists find ways of artificial radioactive decay of atoms of stable periodic table elements. Rutherford in 1919 showed that alpha-ray bombardment on nitrogen produces an isotope of oxygen and proton. Apart from the above natural or artificial radioactivity, nuclear fission and nuclear fusion reaction are examples of the other two types of nuclear reactions. In learning chemistry, nuclear reactions are different from chemical reactions in many respects. The outer orbital electrons have participated in the chemical reaction and atomic nuclei have participated in the nuclear reaction. The working process of nuclear fission and fusion reactions is given below the picture,
Nuclear Reaction Types
Bohr in 1936 suggested that nuclear reactions are two-stage processes. The atomic number of the produced nucleus must be the sum of the atomic number of target and projectile atoms. The produced nucleus subsequently splits down into new products that are energetically feasible.
The mass number of the compound nucleus is the sum of the mass numbers of the initial particles and also the sum of the mass numbers of final products in a nuclear reaction. However, the total atomic masses would not remain constant.
When the total atomic mass of the products is less than the initial participants of nuclear reactions, energy will be given out. It may be calculated from the Einstein equation, E = mc2.
Examples of Artificial Transmutation
In 1934, Irene and Frederick Joliot-Curie studied the artificial transmutation reaction for chemical elements. By bombardment of alpha particles on certain light isotopes produces several radioactive isotopes of low mass number.
For example, aluminum target bombardment by alpha particles produces a phosphorus isotope by artificial transmutation.
Not only alpha particles but other accelerated particles like protons, neutrons, and deuterons are extensively used to obtain artificial radioactive elements. Some examples of nuclear reactions are given below the table,
| Projectile |
Transmutation |
Type |
| Alpha |
3Li7 + 2He4 → 5B10 + 0n1 |
α, n |
| 5B11 + 2He4 → 7N14 + 0n1 |
| Neutron |
13Al27 + 0n1 → 11Na24 + 2He4 |
n, α |
| 26Fe56 + 0n1 → 25Mn56 + 1H1 |
n, p |
| 19K39 + 0n1 → 19K38 + 20n1 |
n, 2n |
| Proton |
3Li7 + 1H1 → 22He4 |
p, α |
| 6C12 + 1H1 → 7N13 + γ |
p, γ |
| Deuterium |
13Al27 + 1D2 → 14Si28 + 0n1 |
d, n |
| 4Be5 + 1D2 → 4Be10 + 1H1 |
d, p |
| Gamma rays |
1H2 + hν → 1H1 + 0n1 |
γ, p |
| 6C12 + hν → 4Be8 + 2He4 |
γ, α |
Artificial radioactive elements behave in the same fashion as natural radioactive elements. The radioactive decay of these elements follows the first-order rate law of chemical kinetics.
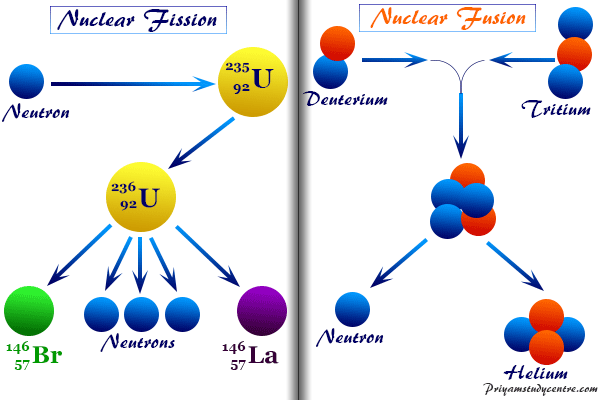
Nuclear Fission and Fusion
For more details about nuclear fission of nuclear fusion, read the following topics,
Nuclear Fission
All the artificial and natural nuclear reactions involved small changes in mass or atomic number. These changes either increase mass by the absorption of particles or decrease mass by the emission of particles.
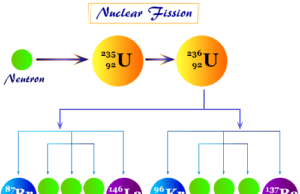
The disintegration of heavy nuclei breaks up into two nuclei of comparable masses is called nuclear fission. The energy released in the fission of nuclei originates from the loss of mass occurring in the process.
Nuclear Fusion
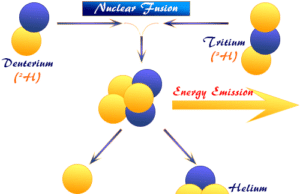
On the other hand, if the light nuclei were joined or fused together, there would be a loss of mass. Such a fusion process would lead to the liberation of energy. For example, if two protons and two neutrons are fused in a nuclear reaction, the loss of mass is converted into energy which is very high.