Atomic Theory Multiple Choice Questions
Atomic theory multiple choice questions or quizzes set in online learning chemistry for school college students provide 9 practice problems with their answers. These questions are related to modern atomic structure, the Bohr model of hydrogen atom, electromagnetic spectrum, quantum numbers orbitals diagram. According to the Rutherford model of an atom, the electron particles revolve around the nucleus in a constant motion. However, a moving charged particle emits radiation by losing kinetic energy and finally falls into the nucleus. To overcome the objection raised against Rutherford’s model, Neils Bohr put forward several novel postulates. However, the modern structure of atoms or molecules is discussed on the basis of electronic configuration, chemical bonding, and the position of elements on the modern periodic table.
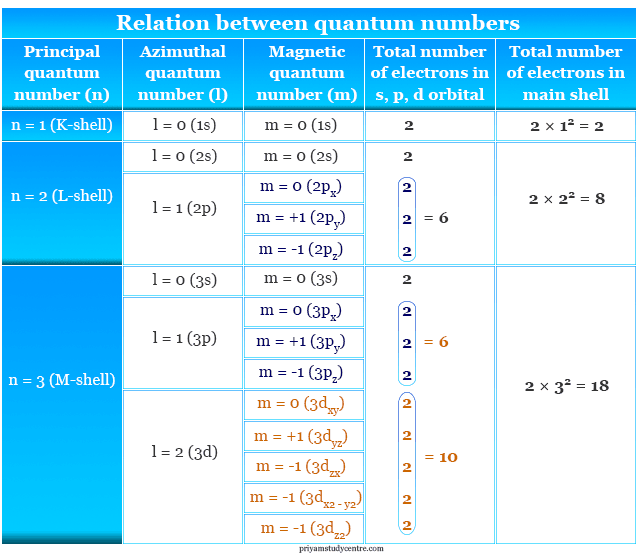
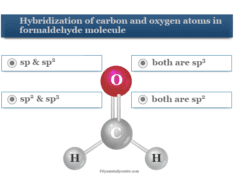
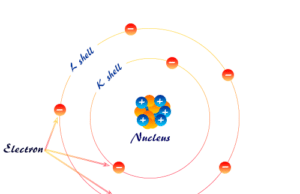
In the above picture, we can discuss the different quantum numbers that help to solve such atomic theory MCQs. Therefore, start the below quiz and evaluate yourself,