Acids and Bases
Acids and bases in chemistry can be defined by five different theories or concepts. Most commonly we can define, acids are chemical substances that are sour in taste produces hydrogen ion (H+) in water solution, and turn blue litmus to red. Similarly, bases are chemical substances that are bitter in taste, soapy to the touch, produce hydroxyl ion (OH−) ion in water solution, and turn red litmus to blue. Examples of some naturally occurring acids are acetic acid (vinegar), citric acid (orange and lemon), tartaric acid (tamarind), oxalic acid (tomato), methanoic acid (ant sting), folic acid (vitamin B9), ascorbic acid (vitamin C), etc. Examples of some commonly used mineral acids or inorganic acids are hydrochloric acid, nitric acid, phosphoric acid, sulfuric acid, boric acid, etc.
The sour taste of some common fruits (unripe mango, lemon, orange, and tamarind) suggests that they are acidic in nature. These contain various organic acids that change the taste of such fruits. Amino acids are important biomolecules that are required for the synthesis of protein which helps to repair and grow human and animal bodies. These are the biological substances that contain acidic (carboxyl) and basic (amine) groups in their structure.
Sodium hydroxide (NaOH) and potassium hydroxide are the most common and useful bases that we use widely in laboratories and chemical industries. The other bases are lithium hydroxide (LiOH), milk of magnesia [Mg(OH)2], calcium hydroxide [Ca(OH)2], ammonium hydroxide, etc.
pH of Acids and Bases
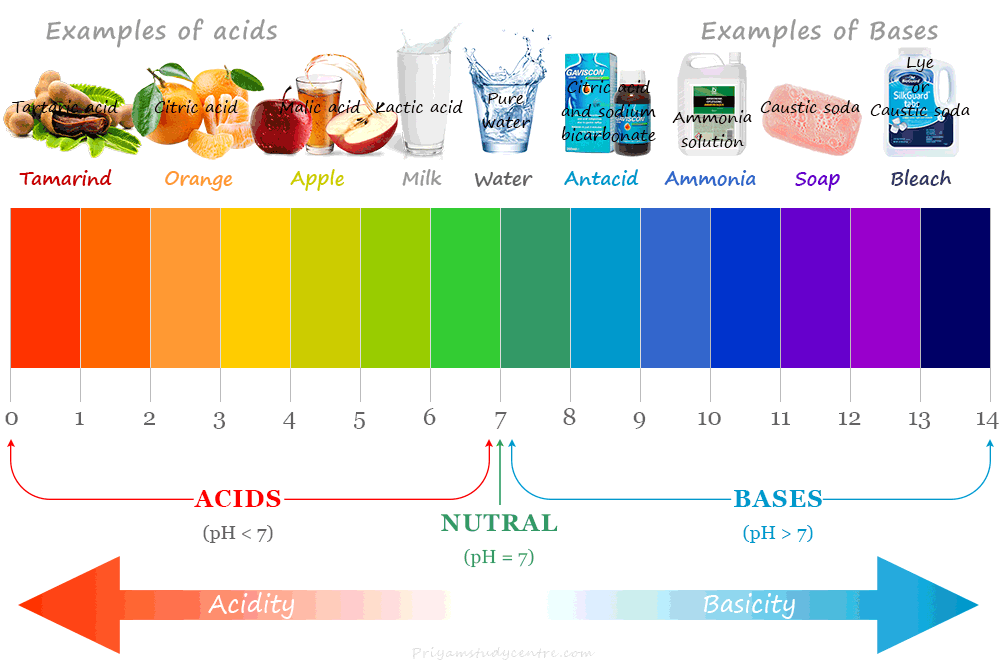
The pH scale is used for measuring hydrogen ion concentration in an aqueous solution. It is a number that indicates the acidic or basic nature of a solution. The higher the hydrogen ion concentration lower its pH value.
Pure water is neutral because of the absence of free hydrogen ions in pure water. Present day, a pH meter is used widely to determine the pH value of various substances. The measurement of pH values identifies acids and bases in the following ways:
- When pH > 7, the solution is basic in nature
- If pH = 7, the solution is neutral in nature
- When pH < 7, the solution is acidic in nature
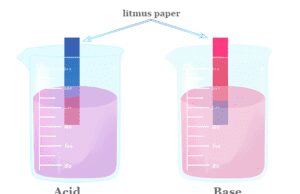
We can also check the acidic and basic nature of substances by litmus paper. It can change its colour from blue to red in an acidic solution and red to blue in a basic solution. Therefore, it is easily used for the identification of acidic or basic solutions.
Many natural and synthetic indicators can change their colour or odour in a specific pH range. Methyl orange and phenolphthalein are examples of such kind of indicators which are used mainly during acid-base titration in laboratories.
Definition of Acids and Bases
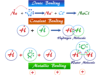
Acids and bases are defined by various other theories and concepts. These theories and concepts may include:
- Arrhenius Theory
- Bronsted-Lowry theory (conjugate acid-base pair)
- Solvent System Concept
- Lewis Acid-Base Theory
- Hard Soft Acid Base Principle (HSAB)
A full discussion of these different theories or concepts of acids and bases is discussed in various topics of learning chemistry. In this part, we only study the definition of acids and bases by these various theories or concepts.
Arrhenius’s Definition of Acids and Bases
According to the Arrhenius definition, an acid is any hydrogen−containing substance that gives an H+ ion in the aqueous solution whereas a base gives an OH− ion in the aqueous solution.
Protonic Concept (Bronsted-Lowry Theory)
Bronsted and Lowry in 1923 independently proposed a more general definition of acids and bases in terms of proton or hydrogen ion (H+).
- According to Bronsted-Lowry theory, an acid is a substance that donates a hydrogen ion (H+) ion or a proton to form its conjugate base.
- Similarly, a base is a substance that accepts a hydrogen ion (H+) or a proton to form its conjugate acid.
Acid-base pairs are formed from each other mutually by the loss or gain of a proton. Therefore, when sulfuric acid loses one hydrogen ion or proton, it forms its conjugate base hydrogen sulfate (HSO4−) anion. Similarly, water can accept one proton to form its conjugate acid hydronium ion (H3O+)
Solvent System Concept
Solvent system definitions are extended forms of protonic definitions of acids and bases occurring in non-aqueous solvents containing hydrogens.
- According to the solvent system concept, an acid is a solvent cation or any substance that increases the concentration of the solvent cations normally produced during the autoionization of solvent. For example, ammonium chloride (NH4Cl ) in ammonia solution.
- Similarly, a base is a solvent anion or any substance that increases the concentration of the solvent anions normally produced during the autoionization of the solvent. For example, potassium amide (KNH2) in ammonia solution.
Examples of non-aqueous solvents containing hydrogens are ammonia (NH3), hydrogen fluoride (HF), sulfuric acid (H2SO4), acetic acid (CH3COOH), hydrogen cyanide (HCN), and alcohol.
Lewis Acid-Base Theory
Lewis’s theory explains the acid-base phenomena not in terms of ionic reactions but in terms of the electronic structure of acid and base along with the formation of a coordinate covalent bond.
- A Lewis acid is a chemical species that contains an empty orbital that is capable of accepting one electron pair from a Lewis base to form a coordinate covalent bond.
- Similarly, a Lewis base is a chemical species that has the ability to donate an electron pair to a given Lewis acid for the formation of a coordinate covalent bond.
Therefore, a Lewis acid and Lewis base formed a product that contain a coordinated covalent bond. For example, ammonia can donate, its free electron pair to a hydrogen ion (H+) to form an ammonium ion (NH4+). It contains a coordinated covalent bond between one hydrogen and an ammonia molecule.
Hard Soft Acid Base Principle (HSAB)
It explains the stability of chemical complexes formed by hard and soft acids and bases. The definitions of these hard and soft acids and bases are:
- Hard Acid: Like a hard base, a hard acid is difficult to polarize. For example, a cationic hard acid Al+3 has a small size, high positive charge, and a noble gas electronic configuration.
- Hard Base: A hard Lewis base is one in which the donor atom has low polarizability and high electronegativity.
- Soft Acid: Like a soft base, a soft acid is readily polarized. Therefore, a soft acid has a large size, low positive charge, and does not contain noble gas electronic configuration.
- Soft Base: A soft Lewis base is one in which the donor atom is easily polarized and has low electronegativity.
The hard soft acid base (HSAB) principle states that a stable complex is formed when a hard acid reacts with a hard base or soft acid reacts with a soft base.
Properties of Acids and Bases
During the neutralization reaction, acids react with bases to produce salt and water.
Acid (HX) + Base (MOH) → Salt (MX) + Water (HOH)
Therefore, acids and bases have common properties of neutralization reaction.
Both acids and bases conduct electricity in their aqueous solutions due to the presence of free ions. Along with these, they have various individual properties.
Properties of Acids
Most of the mineral or inorganic acids are corrosive in nature, have low pH values (less than 7), turn blue litmus to red, and are sour in taste. They also have some chemical properties. They react with metals, metal carbonate or bicarbonate, and basic metal oxides.
Reaction with Metals:
Acids, like dilute H2SO4 and dilute HCl, react with certain active metals such as zinc (Zn) and iron (Fe) to form salt and evolved hydrogen gas. Therefore, these acids or substances containing such acids should not kept in metal containers.
Metal + Dilute acid → Salt + Hydrogen gas
Zn + H2SO4 → ZnSO4 + H2↑
In the above reaction, zinc reacts with dilute sulfuric acid to form zinc sulfate and evolved hydrogen gas.
Reaction with Metal Carbonate:
Acids react with metal carbonate or bicarbonate (hydrogen carbonate) to produce their corresponding salts, carbon dioxide, and water.
Carbonate + Acid → Salt + Carbon dioxide + Water
CaCO3 + 2HCl → CaCl2 + H2O + CO2↑
Reaction with Metal Oxide:
An acid reacts with a metal oxide that is basic in nature to form salt and water.
Basic metal oxide + Acid → Salt + Water
CuO + 2HCl → CuCl2 + H2O
Properties of Bases
Bases are those chemical substances that are bitter in taste, soapy in touch, have high pH values (pH > 7), and turn red litmus to blue. Strong bases also react with metal and non-metallic oxides.
Reaction with Metals:
Strong bases react with active metallic elements such as aluminum (Al) and zinc (Zn) to produce hydrogen gas. Therefore, strong bases would not be kept in active metal containers.
Metal + Base → Salt + Hydrogen gas
Therefore, strong bases such as sodium hydroxide (NaOH) or potassium hydroxide (KOH) react with zinc to produce sodium zincate (Na2ZnO2) or potassium zincate (K2ZnO2) and hydrogen gas.
Zn + 2NOH → Na2ZnO2 + H2↑
Zn + 2NOH → K2ZnO2 + H2↑
Reaction with Acidic Oxides:
Bases react with non-metallic oxides to produce salt and water.
Base + Non-metallic oxide → Salt + Water
CO2 + Ca(OH)2 → CaCO3 + H2O
The above reaction shows that non-metallic oxides are acidic in nature because bases react commonly with acidic oxides.
Difference between Acids and Bases
The most common differences between acids and bases are:
| Acids | Bases |
| Acids (H2SO4, HCl, HNO3, etc.) give hydrogen ions when dissolve in water. | Bases (NaOH, LiOH, KOH, etc.) give hydroxyl ions when dissolved in water. |
| They are sour in taste. | They are bitter in taste and soapy in nature. |
| Acids turn blue litmus paper to red colour. | Bases are chemical substances that turn red litmus paper to blue colour. |
| The pH value of acids is less than 7. | The pH value of bases is greater than 7. |
Uses of Acids and Bases
Acids and bases are the most common chemical substances that we see and use widely in our daily lives. The uses of acids and bases in industry and daily life are given below in the topics.
Uses of Acids
Acids are used widely for the preservation and production of various chemicals and food products. The uses of most common acids in our daily lives and industries may include:
- Acetic acid: A diluted solution of acetic acid or vinegar has various household applications. It can be used widely for cooking and preservation of foods, baking, cleaning, and weed control.
- Citric acid: Found in lemon and orange is an insecticide and disinfectant to help destroy bacteria and viruses. It can be used mostly for preserving and marinating meats, and flavor foods and beverages.
- Sulfuric acid: It is an important mineral acid used for manufacturing our daily items such as dyes, glue, wood preservatives, and automobile batteries. It is also used in chemical industries for making fertilizer, paints, rayon film, etc.
- Phosphoric acid: It is used for making our daily items such as soaps, waxes, polishes, and detergents. It may also used in the chemical industry for the production of superphosphate fertilizers, livestock feeds, phosphate salts, and polyphosphate compounds.
- Boric acid: It is typically utilized in industrial processing and manufacturing but is also used as an additive for the production of pharmaceutical products, cosmetics, lotions, soaps, mouthwash, toothpaste, astringents, and eyewash.
Uses of Bases
Like acids, bases are also important chemical substances that are used for the production of various chemical products and daily needed items. The uses of most common bases may include:
- Sodium Hydroxide: Sodium hydroxide (NaOH) is an important chemical that is used widely for the manufacturing of soap, paper, and rayon.
- Calcium hydroxide: Slaked lime or calcium hydroxide [Ca(OH)2] is used to manufacture bleaching powder (a good disinfectant agent). It is also used in food and paper industries for the production of paper and processing of food items. The soil pollution caused by acid rain and high use of fertilizer can be reduced by employing slaked lime in acidic soil.
- Magnesium hydroxide: HCl is present in the stomach and helps in the digestion of food that we eat. Milk of magnesia or magnesium hydroxide is a medicine or antacid that reduces the effect of acidity caused by producing too much acid in your stomach during indigestion.
- Ammonium hydroxide: It is a common commercial form of ammonia that is used widely in laboratories and chemical industries for making household cleaner, treatment of water, food production, furniture darkening, etc.
Frequently Asked Questions – FAQs
What acids are in the stomach?
A very small amount of hydrochloric acid (HCl) is present in the stomach and helps in the digestion of food. Other components found in the stomach include potassium chloride (KCl) and sodium chloride (NaCl). During indigestion, the stomach produces too much acid which causes pain and irritation.
What acids are in tomatoes?
Tomato contains more than 10 types of organic acids such as citric acid, malic acid, ascorbic acid (vitamin C), oxalic acid, etc.
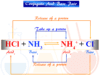
What happens when acids and bases are mixed?
When acids and bases are mixed they undergo neutralization reactions to form salt and water.
Acid + Base → Salt + Water
HNO3 + KOH → KNO3 + H2O
HCl + NaOH → NaCl + H2O
How to identify acids and bases?
Acids and bases are most commonly identified by litmus paper. An acid can change the coluor of blue litmus paper to red and a base can change the colour of red litmus to blue. The pH value of acids is less than 7 but the pH value of bases is greater than 7.
Do acids and bases conduct electricity?
An acid or a base molecule dissociates in an aqueous solution to produce H+ and their respective anion or OH− and their respective cation. These free ions carry electrical charge from one place to another and conduct electricity.
How much acid is in coffee?
Coffee contains many acids with an average pH level of 4.85 to 5.10.
What are acids and bases in chemistry?
In chemistry, acids are those substances that are sour in taste and give H+ ions in the presence of water. Similarly, acids are those substances that are bitter in taste and give OH− ions in the presence of water.