Polarimetry Principle
Polarimetry principle in analytical chemistry is a method for measuring the rotatory power or optical rotation of a substance by the instrument polarimeter. Many transparent non-symmetrical optically active molecules can rotate the plane of polarized light or electromagnetic radiation. The principle of polarimetry or optical rotation depends upon various factors such as the number of molecules in the path of electromagnetic radiation, concentration, length of containing vessel, wavelength, and temperature. The wavelength used in the polarimetry instrumentation is specific. Commonly, we used a sodium D line with a wavelength of 589 nm. The instrumentation of polarimetry in a polarimeter is given below the diagram,
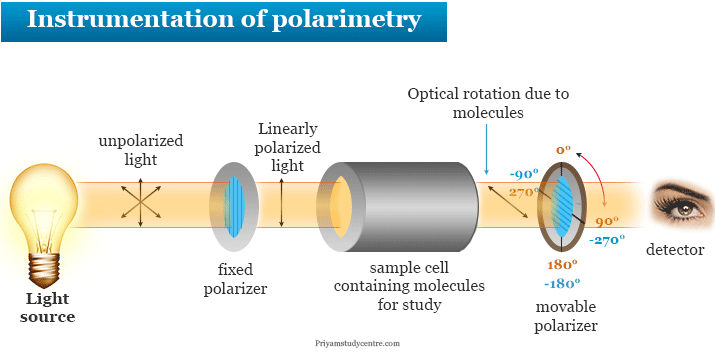
Instrumentation of Polarimetry
Polarimetry measures the angle of rotation by passing polarized light through an optically active (chiral) substance with an instrument like a polarimeter. Essentially, a polarimeter contains two Nicol prisms, one is the polarizer and the other is the analyzer. The polarimetry instrumentation contains the following components,
Unpolarized Light Sources
All common sources of light obtained from the Sun, incandescent or fluorescent lights, and flame are examples of unpolarized light. Natural light obtained from different sources is slightly polarized due to scatterings and reflections.
In Polarimetry instrumentation, we used monochromatic radiation from a sodium lamp with a wavelength of 589 nm for sources of light.
Fixed or Linearly Polarizer
It is a Nicol prism made of calcite. In polarimetry, Nicol prism or fixed polarizer converts unpolarized light to a linearly polarized light. It can convert a linearly polarized signal to a circularly-polarized signal in a clockwise or counterclockwise direction.
Sample Cell
It contains optically active molecules for study. These optically active molecules rotate the plane of polarized light due to their optical activity. There are two types of optical rotation of light found in the sample cells.
- When the molecule present on the sample cell rotates the plane-polarized light in the clockwise direction, we called it dextrorotatory rotation.
- However, the molecules rotate the light counterclockwise, we call it levorotatory rotation.
Movable Polarizer
Analyzing rotator Nicol prism also made by calcite. The analyzing rotator prism is fixed in such a way that it can be rotated easily about the axis of the incident light. It is used to measure the rotatory power or angle of rotation of a particular optically active compound.
Detector
The detector is a device or instrument used to detect the presence of a particular object or substance. In a polarimeter instrument, we used an eye or photoelectric cells as a detector that detects the optical rotation of light.
Polarimetry Optical Rotation
The polarimetry principle is used to measure the optical rotation of polarized light which passes through optically active compounds like quartz, sugar, etc.
The specific rotation,
[α] tD = 100α/lc
where α = observed rotation;
l = thickness of the layer in decimeters;
c = concentration of the substance
The determination is carried out at a temperature of t °C using a sodium D line. The specific rotation of some optically active compounds is given below the table,
| Specific rotation | |
| Compound | [α]D20 |
| d-glucose | +52.7 |
| d-fructose | -92.4 |
| maltose | +130.4 |
| sucrose | +66.5 |
| L-tartaric acid | +14.1 |
Applications of Polarimetry
- Many transparent non-symmetrical organic compounds such as tartaric acid, sucrose, and glucose contain stereoisomers. A polarimetry principle in the polarimeter can be used to identify the isomer present in the sample. The isomer is dextro-rotatory if it rotates polarized light in a clockwise direction and the isomer is Levo-rotatory if it rotates polarized light in a counterclockwise direction.
- Many chemicals exhibit specific types or specific values of optical rotation. Therefore, the polarimetry principle can be used to identify unknown samples based on their specific rotation.
- Specific rotation data is also used to calculate the concentration and purity of industrial chemicals.
- We used polarimetry for the analysis of antibiotics and enzymes. The antibiotics can be destroyed by enzymes. Therefore, the greater the enzyme concentration, the more the destruction of antibiotics.
- The concentration and purity of food, beverage, and pharmaceutical products can be done by the polarimetry principle or instrumentation.