Concentration of a Solution
Concentration in chemistry is a calculation of how much substance is present in a chemical solution or mixture. In learning chemistry, the concentration of a solution is defined as the amount of solute dissolved in a given amount of solvent. There are many ways to calculate the concentration of a solution used for different purposes. Therefore, it can be expressed in different units or measurements. The most common units that we used to calculate the concentration of a solution in chemistry are normality, molality, morality, mass percent, volume percent, and mole fraction.
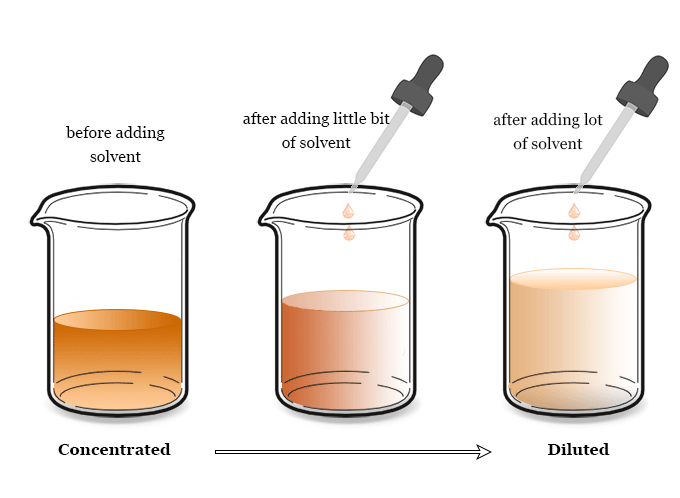
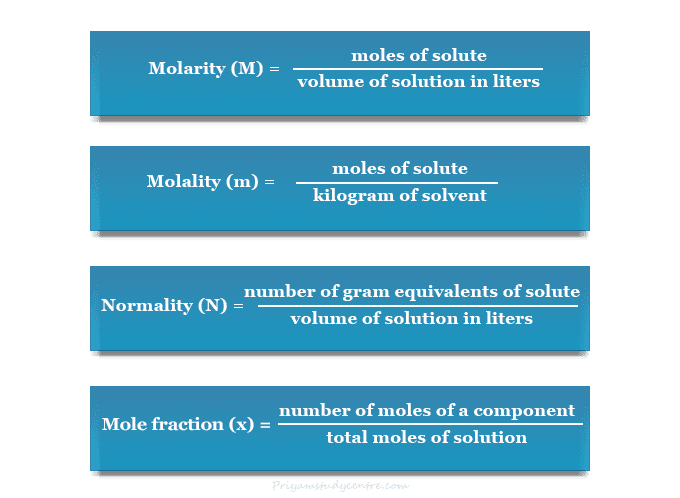
Concentration Calculation Formula
The concentration calculation formula for molarity, morality, normality, and mole fraction is given below the picture,
Molarity Calculation
Molarity (also called molar concentration) is the most common unit of concentration that can be calculated by how many g-moles of solute are present in 1 liter of solution. It can be derived by the symbol M.
The molarity of a solution may be expressed by units g-mol L−1 or g-mol dm−1. If 0.1 g mole of NaOH is present in one liter of solution, the molarity of such solution = 0.1M.
Molar Solution
A molar solution is a solution that contains one gram mole of solute per liter of a solution.
- If 40 g or one mole of NaOH is present per liter of a solution, the molarity of such solution = 1 or 1M.
- If 98 g or one mole of H2SO4 (sulfuric acid) is present per liter of a solution, the molarity of such solution = 1 or 1M.
| Number of g mol of solute per liter of solution | Name of the solution | Symbol | Molarity |
| 1 gram mole | molar | M | 1 |
| x-gram mole | x-molar | xN | x |
| 0.5 gram mole | semimolar | 0.5N | 0.5 |
| 0.1 gram mole | decimolar | 0.1N | 0.1 |
| 0.01 gram mole | centimolar | 0.01N | 0.01 |
Molality Calculation
A molality is a unit of concentration that can be calculated by how many g-mol of solute are present in 1 kg of solvent. It can be expressed by the symbol m.
For example, if 0.1 g mol of urea is dissolved in 1 kg of alcohol, the molality of such solution = 0.1m.
The density of water is approximately 1 kg/L at room temperature. Therefore, M and m are nearly the same for the aqueous solution.
Formality in Chemistry
Some ionic compound such as sodium chloride (NaCl), potassium chloride (KCl), sodium carbonate (NaCO3), and calcium chloride (CaCl2) dissociates completely to form cations and anions in water. There is no existence of such chemical species in an aqueous solution. We used formality instead of molarity to express the concentration of such ionic compounds in solution.
In chemistry, both molarity and formality are the units of concentration. The formality of a solution can be calculated by the formula mass of any solute dissolved in one liter of solution.
Normality Calculation
The normality of a solution is the number of gram or mole equivalents of a solute present in one liter of the solution. It can be expressed by the letter N.
It may be expressed by unit g-equiv L−1 or g-equiv dm−1. Normality or equivalent concentration calculation is used in the following chemical reactions,
- Normality is used to express the concentration of hydronium ions or hydroxide ions in acid-base chemistry.
- In a redox reaction, the normality can describe the number of electrons accepted or donated by atoms or ions.
- In a precipitated reaction, normality is used to calculate the amount of precipitated ion.
Normality Formula
The normality of a solution can be calculated by the following formula,
Normality (N) = W/(E × V)
Where W g of solute present in V liter of solution
The equivalent weight of solute = E.
Example
What is the normality of a solution of 160 grams of NaOH (sodium hydroxide) in 2000 ml of water?
If 160 g NaOH is present in 2 liters of water, the normality of such solution from the above formula,
= 160/(40 × 2)
= 2
Normal Solution
A normal solution is such a solution where one gram equivalents of solute are present in one liter of a solution.
- If 40 g or one gram equivalent of NaOH is present per liter of a solution, the normality of such solution = 1 or 1N.
- If 49 g or one gram equivalent of H2SO4 (sulfuric acid) is present per liter of a solution, the normality of such solution = 1 or 1N.
| Number of gram equivalent of solute per liter of solution | Name of the solution | Symbol | Normality |
| 1 gram equivalent | normal | N | 1 |
| x-gram equivalent | x-normal | xN | x |
| 0.5 gram equivalent | seminormal | 0.5N | 0.5 |
| 0.1 gram equivalent | decinormal | 0.1N | 0.1 |
| 0.01 gram equivalent | centinormal | 0.01N | 0.01 |
Normal and Molar Solution
A normal solution is a chemical solution that contains one gram equivalent solute per liter of the solution but a molar solution contains one mole of solute per liter of solution.
The normal and molar solutions of some common chemical compounds are given below in the table,
| Solute | Molecular weight of solute | Equivalent weight of solute | Volume of Solution | Concentration | |
| Molarity | Normality | ||||
| Hydrochloric acid (HCl) | 36.5 g | 36.5 g | 1 liter or 1000 ml | 1M | 1N |
| Sulfuric acid (H2SO4) | 98 g | 49 g | 1 liter or 1000 ml | 1M | 1N |
| Sodium carbonate (Na2CO3) | 106 g | 53 g | 1 liter or 1000 ml | 1M | 1N |
| Sodium hydroxide (NaOH) | 40 g | 40 g | 1 liter or 1000 ml | 1M | 1N |
| Potassium hydroxide (KOH) | 74.6 g | 74.6 g | 1 liter or 1000 ml | 1M | 1N |
The molecular weight and equivalent weight of hydrochloric acid, nitric acid, sodium hydroxide, and potassium hydroxide are similar. Therefore, the normal and molar solutions of such compounds are similar.
Mole Fraction Calculation
Mole fraction or molar fraction is a way to derive concentration. It may be calculated by the number of moles of one component present in a solution divided by the total number of moles of all chemical components. The amount of every component can be measured by mole number.
Let the mole number of solute and solvent in a solution equal to n1 and n2 respectively.
Mole fraction of solute,
x1 = n1/(n1 + n2)
Mole fraction of solvent,
x2 = n2/(n1 + n2)
Hence, x1 + x2 = 1. Therefore, the sum of the mole fractions of solute and solvent = 1.
Problem: How to find the mole fraction of 2 moles of salt dissolved in 144 grams of water?
Solution: 180 grams of water = 144/18 = 8 moles of water.
Therefore, the mole fraction of salt,
= 2/(2 + 8) = 1/5 = 0.2
Percentage Concentration Calculation
Volume Percent Concentration
The volume percent is used to calculate the concentration of a solution when the volume of a solute and the volume of a solution is known.
Mathematically, volume percent = (volume of solute/volume of solution) × 100%.
For example, 10% (v/v) aqueous ethanolic solution means, 10 mL of pure ethanol dissolved in 100 mL of water.
Problem: Calculate the volume percent of 5.0 milliliters of ethanol when diluted by water to give a 125 milliliter solution.
Solution: From the above formula, the volume percent of ethanol = (5/125) × 100%
= 4%.
Mass Percent Concentration
The mass percent is used to calculate the concentration of a solution for the known mass of the solute and solution.
Mathematically, mass percent
= (mass of solute/mass of solution) × 100%.
For example, 70% (w/w) HNO3 solution means, 70 g of pure HNO3 dissolved in 100 g of a solution.
The Nichrome alloy contains 75% nickel, 12% iron, 11% chromium, and 2% manganese by mass. How to calculate the amount of iron in 200 grams of Nichrome alloy?
The concentration of iron in Nichrome alloy is expressed in mass percent. Therefore, 100 grams of Nichrome contains 12 grams of iron.
Hence the amount of iron in 250 grams of Nichrome alloy,
= (12/100) x 200
= 24 g
Mass/Volume Percent Concentration
It is another version of a percentage concentration. The mass/volume (w/v) percent is used to calculate the concentration of a solution when the mass of a solute and the volume of a solution is known.
Mathematically, mass/volume percent
= (mass of solute/volume of solution) × 100%.
For example, 0.9%( w/v) NaCl solution in medical saline means, 0.9 g of pure NaCl present in 100 ml of a solution.
Parts Per Million (ppm) Meaning
Parts per million (PPM) is a unit of concentration used for expressing a very dilute solution in science and chemical engineering. ppm meaning how many parts of solute are dissolved in one million parts of a solution.
It is used mainly to calculate the concentration level of pollutants in the air, water, and other fluids.