Electromagnetic Radiation and Waves
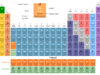
Electromagnetic radiation in physics or chemistry describes the form of energy that is transmitted through space at an enormous velocity. Each type of electromagnetic radiation has both the properties of waves and particles. Gamma rays, x-rays, ultraviolet (uv), visible, infrared (ir), microwave, and radio frequency are examples of electromagnetic radiation which contain a definite region of the spectrum. We used wavelength and frequency to describe different types of electromagnetic spectrum radiation.
Electromagnetic Wave
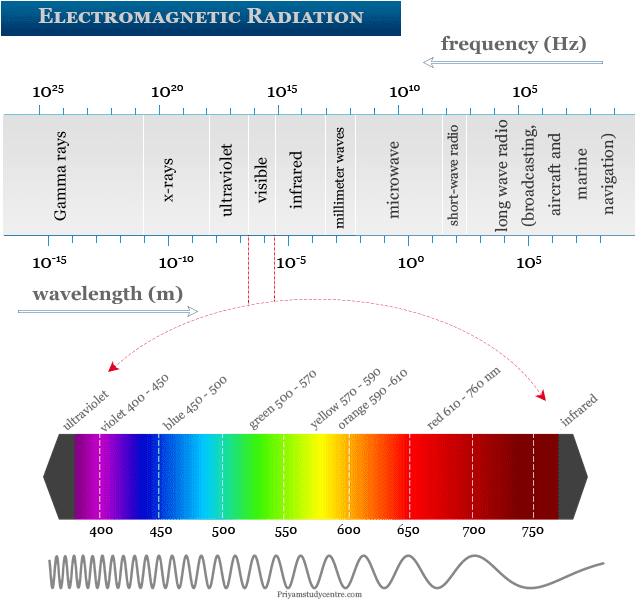
An electromagnetic wave has electric and magnetic components. The two components in electromagnetic radiation oscillate in the plane perpendicular to each other.
From the above picture, the vector representation of electromagnetic waves moving along the x-axis. The electric field varies in the direction of the y-axis and the magnetic field H varies in the direction of the z-axis.
The velocity of electromagnetic waves in a vacuum is independent of frequency. In comparison to other wave phenomena such as sound, the electromagnetic wave does not require any supporting medium for its transmission.
An electromagnetic wave can be characterized by the following parameters such as wavelength, frequency, wavenumber, and velocity.
Wavelength of Electromagnetic Radiation
The wavelength of electromagnetic radiation is the distance between two successive maxima of an electromagnetic wave. It is denoted by the Greek letter Lambda (λ). The usual units of wavelength vary with the region of electromagnetic spectrum radiation.
The more commonly employed units of wavelength are meter (m), centimeter (cm), millimeter (mm), micrometer (μm), nanometer (nm), and angstrom.
| 1 μm | 10−4 cm | 10−3 nm | 10−6 m |
| 1 nm | 10−6 μm | 10−7 cm | 10−9 m |
| 1 Å | 10−8 cm | 0.1 nm | 10−10 m |
Frequency of Electromagnetic Radiation
The number of complete wavelengths passing through a given point in a unit of time is called the frequency of electromagnetic radiation. It is denoted by the Greek letter ν. Frequency is the unit of reciprocal of time.
The frequency of electromagnetic radiation is generally expressed by cycles per second or Hertz (Hz) or Fresnel. By their definitions, wavelength and frequency are inversely proportional to each other.
| 1 Hz | 1 cycle s−1 | |
| 1 Fresnel | 1012 Hz | |
| 1 MHz | 103 kHz | 106 Hz |
Wavenumber
Frequency is more fundamental than the wavelength of electromagnetic waves. It is defined as the number of waves per centimeter in a vacuum.
Mathematically, wavenumber = 1/wavelength (λ)
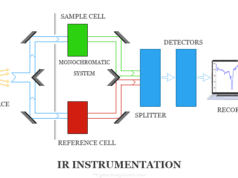
The wavenumber is generally used in infrared (IR) spectroscopy.
Velocity of Electromagnetic Waves
The velocity is dependent on the medium through which radiation passes. It has the unit of cm s−1 or m s−1.
The product of the wavelength and frequency of electromagnetic radiation is equal to the velocity of the wave in the medium.
∴ Wavelength × frequency = velocity
or, λ × ν = c
When the density of the medium through which electromagnetic radiation passes decreases, the velocity, and wavelength of radiation increase. It reaches the maximum or constant value in a vacuum. Therefore, electromagnetic waves in a vacuum travel with a constant velocity of 3 x 108 m s−1.
Electromagnetic Energy
The wave nature of electromagnetic radiation fails to explain the phenomena like photoelectric effect. To explain such phenomena, it is assumed that electromagnetic radiation contains a stream of discrete particles of pure energy. These streams of discrete particles are called photons or quanta.
According to Planck’s quantum theory, the energy of the photon is proportional to the frequency of radiation. Electromagnetic energy is given the relationship,
E = hν
Where ν = frequency of electromagnetic radiation
h = Planck constant = 6.62607004 × 10−34 m2 kg/s.
A higher frequency of radiation shows greater photon energy. Therefore, x-rays are more energetic than the rays obtained from visible light.
Example of Electromagnetic Radiation
X-rays, gamma rays, x-rays, ultraviolet, visible, infrared, microwave, and radio waves are examples of electromagnetic radiation that contain a definite region of the spectrum.
Gamma Rays Radiation
Gamma rays are the shortest and most energetic waves in electromagnetic radiation. The main ways for the formation of gamma rays are nuclear fusion and nuclear fission reactions. Most of the gamma waves are generated by nuclear explosions, lighting, and the radioactive decay of substances.
Gamma rays are harmful radiation due to their high penetrating power. It can damage the bone marrow and internal organs of living organisms. Gamma rays are used mainly to sterilize medical equipment, tracers in medicine, and kill cancerous cells in radiotherapy.
X-Rays Radiation
X-rays are the type of electromagnetic radiation which absorbed or emitted by the movement of electrons close to the nuclei of relatively heavy atoms. Energetically, it is similar to radio waves, microwaves, visible light, and gamma rays.
It has enough energy to break down the molecules of living cells. X-rays are used mostly for medical imaging for the detection of bone fractures.
Ultraviolet Radiation
Ultraviolet radiation is non-ionizing radiation obtained mostly from the sun and other sources like mercury vapor lighting, fluorescent, and incandescent lights. It is beneficial for our health because uv radiation from the sun produces vitamin D.
Visible Radiation
Visible radiation or visible light is the form of waves with wavelengths between 400 to 750 nm. The approximate colour of visible light with their corresponding wavelength is given below the table,
| Colour | Wavelength (nm) | |
| Violet | 400 – 450 | |
| Blue | 450 – 500 | |
| Green | 500 – 570 | |
| Yellow | 570 – 590 | |
| Orange | 590 – 610 | |
| Red | 610 – 750 | |
Visible light radiation is used mostly in photography and illumination. In optical fiber communication technology, we also used visible types of radiation transmitted through glass fiber.
Infrared Radiation
Infrared radiation (IR) is a region of the electromagnetic spectrum where wavelengths lie between the visible and microwave regions. The infrared waves are longer than that of visible light but shorter than that of the microwave.
Infrared radiation can be subdivided into three regions,
- Near-infrared
- Mid-infrared
- Far-infrared
All three sub-regions of infrared are part of the electromagnetic spectrum associated with the changes in the vibration of the molecules.
Infrared waves are used mostly in communication technology, spectroscopy, and astronomy. In spectroscopy, it is used for the structure determination of inorganic or organic molecules. It is also used as a source of heat energy in medical and different types of industrial production.
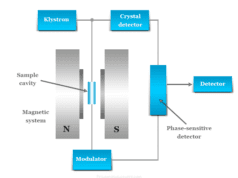
Microwave Radiation
Microwave radiation lies between far infrared and conventional radiofrequency regions of the electromagnetic spectrum. It deals with the pure rotational motion of the molecules. Therefore, it is also called rotational spectroscopy.
For microwave rotation, a molecule must possess a permanent dipole moment. The molecule having a dipole moment generates an electric field that can interact with the electric component of microwave radiation. The non-polar molecules such as hydrogen, chlorine, and methane are microwave inactive.
Microwaves are widely used in modern technology, for the study of various problems in physics, chemistry electronics, and astronomy. Particularly, it is very useful for structure determination.
Microwave radiation is used widely in various communication links such as wireless networks, radio relay networks, radar, satellite and spacecraft, remote sensing, and radio astronomy.
Radio Frequency Electromagnetic Radiation
Radio frequency wave is a part of non-ionizing radiation that arises due to the spin of the nucleus or electron. Such type of electromagnetic radiation is used widely in communication devices such as mobile phones, computers, broadcasting technology, aircraft, and marine navigation.