Definition of Spectrophotometry
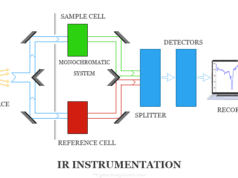
Spectrophotometry is an instrument in the electromagnetic spectrum for the measurement of relative energy (emitted, transmitted, or reflected) as a function of wavelength. A spectrophotometer in electromagnetic spectroscopy is an instrument composed of two units, a spectrometer that produces light of a definite wavelength and a photometer used to measure the intensity of the transmitted or absorbed light. The spectrophotometry instrumentation principle is most commonly applied for ultraviolet visible spectroscopy and infrared spectroscopy. Therefore, a spectrometer is a source for the continuous visible spectrum or a device for obtaining monochromatic light. The most common light source in atomic absorption spectroscopy or spectrophotometry instruments is a tungsten lamp.
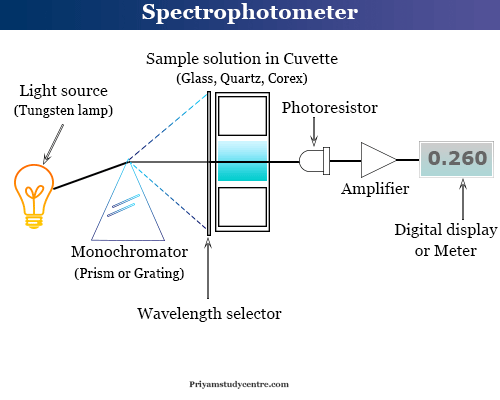
Spectrophotometer Instrumentation
A spectrometer in analytical chemistry works in a way where a sample and reference or blank solution are placed between the spectrometer and photometer. Therefore, it is used to measure the difference in absorption between the sample and the blank or reference solution.
A typical spectrophotometer has the following components,
- Source of light
- Monochromator
- Absorption cell
- Photomultiplier tube
Source of Light
The most common light source in atomic absorption spectrophotometry is a tungsten lamp. Usually, photocurrent depends on the lamp’s voltage.
Photocurrent (i) = K Vn
where V = voltage
n = exponent.
A hydrogen or deuterium lamp is used as the source of light in the ultraviolet region. An important advantage of using a tungsten lamp is the constancy of overall energy output at various wavelengths.
Monochromator
The monochromator is an optical device that separates polychromatic light like sunlight or light obtained from lamps to monochromatic light with narrow bands. Therefore, a prism or grating may be used to separate polychromatic light to monochromatic light
A slit or wavelength selector may be used to pick out the desired wavelength. If the slit is in a fixed position, rotation of the prism or grating is used to obtain the proper wavelength.
Absorption Cell
During the measurement in the visible region, we can use a glass or corex glass cuvette. For measurement in the ultraviolet region, we must use a quartz cell.
The common optical path in spectrophotometry is 10 mm but cells with larger or smaller optical paths are also available. We usually use rectangular cells but cylindrical cells are also available.
Photomultiplier Tube
In spectrophotometry, photomultiplier tubes are extremely sensitive detectors of light in the ultraviolet, visible, and near-infrared regions of electromagnetic radiation. Therefore, it is used as a detector in the spectrophotometer.
Different kinds of materials are used for coating the tube.
| Wavelength (nm) | Coating materials |
| 300 to 500 | sodium |
| 400 to 500 | potassium |
| 250 to 1300 | S-1 (Ag-O-Cs) |
| 200 to 700 | silver-potassium (Ag-K) |
| 200 to 600 | antimony-cesium (Sb-Cs) |
A photomultiplier tube has the following diagram,
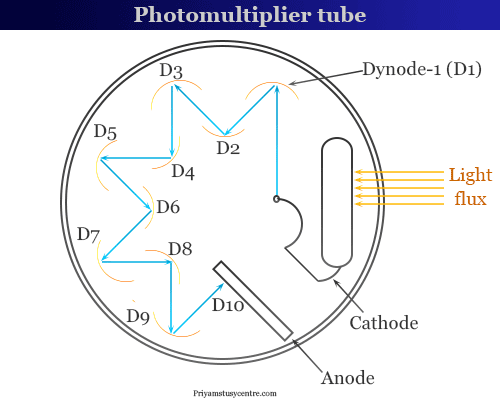
Light falls upon the cell and electron get attacked from different dynodes. From the above diagram, 10 strokes emitted a large number of electrons due to multiplication. It impacts a large current to the anode, which can be directly calculated.
Spectrophotometry Applications
Various types of spectrophotometers and spectrophotometry techniques are used in different fields of science like analytical chemistry physics, biology, biochemistry, and foresic analysis. Some applications of spectrophotometry are given below,
Simultaneous Spectrophotometry
The simultaneous spectrophotometry technique is used to measure the absorbance of a mixture of colored solutions without separating them.
A common example of the application of simultaneous spectrophotometric determination is the analysis of chromium and manganese from a sample of steel without separating the metals.
- On oxidation, manganese gives KMnO4 with an absorption maxima of 545 nm while chromium gives K2Cr2O7 with an absorption maxima of 440 nm.
- Similarly, we can determine palladium and platinum from a mixture by spectrophotometry analysis. Both the metals form complexes with SnCl2 in HClO4 with absorbance maxima at 635 and 405 nm respectively.
We measure the absorbance of the mixture and individual solutions at λ1 and λ2. From these readings, it is possible to construct a simultaneous equation to evaluate the individual concentrations of metals.
Differential Spectrophotometry
Differential spectrophotometry is used in two methods,
- High absorbency method: The high absorbency method is used for the analysis of a very concentrated solution.
- Low absorbency method: Similarly, a low absorbency method is applied for very dilute solutions.
In both techniques, concentration is not altered by external changes.
Reflectance Spectrophotometer
An attachment for making reflectance measurements is available for a standard spectrophotometer. The spectrophotometry reflectance curve provides a series of such measurements given below the diagram,
If desired, reading can be converted to world standard CIE trichromatic values.
For white light, colour of all wavelengths is present in equal intensities. Therefore, the spectral reflectance curve would be a horizontal line. However, the position of the vertical axis depends on the brightness of the sample.
For Pure magnesium oxide (MgO), a horizontal line at any point between 100% T to 0% T would indicate equal absorbance of all wavelengths of radiation in the visible spectrum. It appears gray to the naked eye.
Reflectance spectrophotometry is useful for the measurement of color samples that are ordinarily not soluble in any solvent to give colour solution.
Spectrophotometric Titration
The volumetric titration technique is used for the detection of the endpoint by the eye in the visible region of the spectrum.
- If we use a photometer for the detection of the endpoint, it is called photometric titration.
- When we use a spectrophotometer for the detection of the endpoint, it is termed spectrophotometric titration.
Volumetric titration is classified into four categories
- Acid base titration
- Redox titration
- Precipitation titration
- Complexometric titration
Photometric titration is extensively applied for acid base and complexometric titrations. Therefore, this technique is not very useful for precipitation and redox reactions.
The spectrophotometric technique is used extensively for the analysis of organic compounds but has limited application for non-aqueous solutions.
Light scattering spectrophotometric titration methods in spectrophotometry are also used for the determination of silver from chloride suspension, gold from a gold colloid, potassium with sodium cobalt nitrate, and sodium with zinc uranyl acetate.