What are Enzymes?
Enzymes are the biological catalyst or proteins which are produced in living organisms and speed up the chemical reactions in living cells. There are various types of enzymes produced in our bodies that have a specific biological function. Usually, enzymes are present in various cells and exhibit their activity when they are extracted from their source.
Almost all the metabolic processes are controlled by enzyme catalysis reactions. For example, protease breaks down protein into amino acids which is essential for body growth.
Enzymes are dispersed in water by forming colloids having dimensions in the range of 10−7 to 10−4 cm. All enzymes are globular proteins but many have been identified and a large number have been obtained in crystalline form.
Enzymes Function
Enzymes are proteins that help to speed up the metabolism in our body. Digestion is an imported function of enzymes for the production of energy from foods that we eat. For example, the enzymes from our saliva, pancreas, intestines, and stomach can break down food into fats, proteins, and carbohydrates.
Enzymes also control several body functions like breathing, muscle construction, nerve construction, etc. There are thousands of enzymes produced in our body which has a specific function.
For example, carbohydrase can break down carbohydrates into sugars, lipase can break down fats into fatty acids and protease breaks down protein into amino acids.
Enzymes Classification
The International Commission in 1961 recommended a systematic classification and nomenclature of enzymes. A common method for naming enzymes adds the suffix ase to the name of the substrate.
For example, esterase acts on esters, protease acts on protein, urease acts on urea, etc. According to the International Commission, enzymes are divided into six main groups according to the nature of the enzymolysis reaction.
Oxidoreductases
Oxidoreductases are the types of enzymes that catalyze oxidation reduction reactions in living cells. For example, oxidase is an oxidoreductase enzyme that involves the direct oxidation of molecular oxygen.
Similarly, dehydrogenases involve the removal of hydrogen from the substrate. Other oxidoreductases include peroxidases, hydroxylases, oxygenases, and reductases.
Transferases
Transferases are the class of enzymes that catalyze the transfer of various functional groups from a donner molecule to a receptor molecule.
Hydrolases
Hydrolases are the class of enzymes or biochemical catalysts that catalyze hydrolytic reactions in the biochemical process. They used water to break a covalent bond. Proteases, esterases, and phosphatases are common examples of hydrolases.
Lyases
In biochemistry, lyases are two types. One of which catalyzes the addition of double bonds and the other catalyzes the removal of groups and leaves double bonds from the substrate.
Isomerases
Isomerases are the class of enzymes that catalyze the various types of isomerization. Isomerization is a process in which one isomer is formed from another isomer. Racemases and epimerases are examples of isomerases.
Ligase
In biochemistry, a ligase is an enzyme that catalyzes the formation of a chemical bond between two large types of molecules. For example, DNA ligase catalyzes the DNA fragments by the formation of a phosphodiester bond between two ends of DNA fragments.
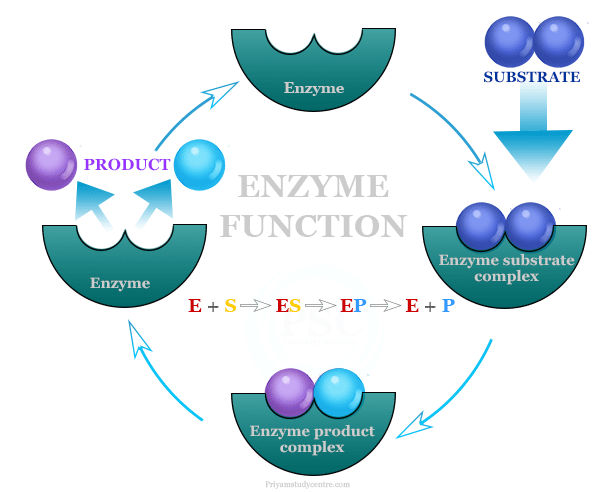
Mechanism of Enzyme Action
The enzyme catalysis is highly specific in nature. For example, the enzyme urease can catalyze the hydrolysis of urea but it does not affect the hydrolysis of methyl urea.
The enzyme catalysis reactions proceed through several steps,
E + S → ES → EP → E + P
The nature of the interaction between enzymes and substrate can be of various types like hydrogen bonding, electrostatic force, hydrophobic bonds, and covalent bonds.
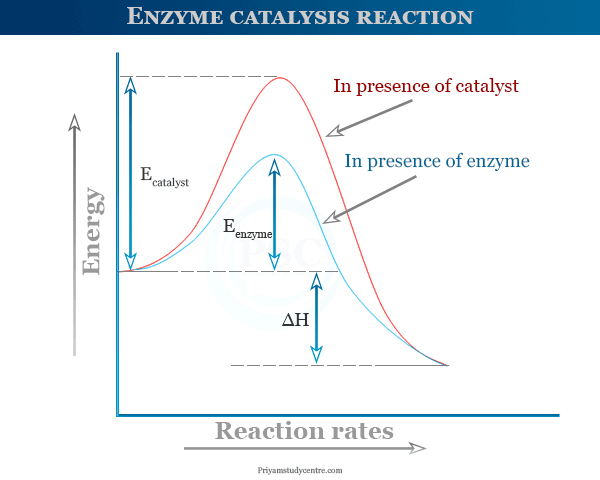
Like chemical catalysts, enzymes also lower the activation energy of reactions that they catalyze.
The efficiency of enzyme catalysis is much greater than the chemical catalyst because they lower activation energy much greater extent. For example, the decomposition of hydrogen peroxide by an enzyme is much more efficient than catalyst platinum.
Factors Affect the Enzyme Activity
The rate of enzyme-catalyzed reactions depends on several factors such as pH, temperature, the concentration of enzyme, and the concentration of substrate.
- The pH of the solution has a great effect on enzyme activity. It has been found that an enzyme behaves efficiently as a catalyst over a narrow pH scale range. It is usually between pH 5 to pH 9.
- Like all chemical reactions, enzymolysis reactions are affected by changes in temperature. Normally, the rate increases as the temperature rises. However, enzymes can be denatured by heat or a too-high temperature. The catalytic activity of the enzyme is destroyed at a high temperature.
- The rate of enzyme-catalyzed reaction depends on the concentration of the substrate and enzyme. If the substrate is excess the rate is directly proportional to the concentration of the enzyme. On the other hand, if the enzyme concentration is kept constant, then the rate increases rapidly as the substrate concentration increases slowly.
Enzyme Inhibitors
Enzyme activity can be reduced or inhibited by the presence of various compounds. These are known as enzyme inhibitors. There are two major types of inhabitation competitive and irreversible.
- In competitive inhabitation, the inhibitor is a compound whose structure and geometry are close to that of the substrate. Competition occurs between two active sites.
- In irreversible inhabitation, the inhibitor forms a highly stable enzyme-inhibitor complex. If sufficient inhibitors are present, the catalytic activity of the enzyme towards the substrate is completely lost.
Cofactors of Enzymes
Many enzymes require the presence of non-protein compounds to perform their catalytic action. These compounds are collectively known as cofactors of activators of enzymes. They are classified into two groups,
- Coenzymes
- Inorganic ions
Coenzymes
Coenzymes are organic compounds that may be separated from enzymes by dialysis.
Inorganic Ions
In some cases, inorganic ions or metal ions are tightly bound to the enzyme. They are called metalloenzymes. In other cases, the enzymes are metal-activated. Metal activators are uni or bivalent cations such as Na+, K+, Mg+2, Zn+2, and Ca+2.
Some enzymes are synthesized in living organisms in an inactive form which is known as a zymogen. For example, the enzyme pepsin is synthesized in living organisms as pepsinogen and it is converted to pepsin in the presence of hydrochloric acid.
Examples of Enzymes
- An enzyme can catalyze a particular type of reaction. For example, esterase hydrolyzes only esters. These are reaction-specific.
- On the other hand, a substrate-specific enzyme may be specific for a particular compound. For example, urease can hydrolyze only urea, phosphatase can hydrolyze only phosphate esters, etc.
Various other examples of enzyme catalysis reactions where starch can be converted into alcohol are given below the picture,
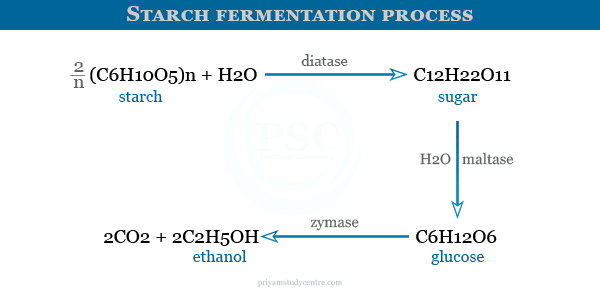
Many enzymes exhibit kinetic specificities such as esterase and pepsin. Although esterase can hydrolyze all the esters. These hydrolyzes can be carried out at different rates. Many enzymes are stereospecific such as maltase hydrolyses α-glycosides but can not function on β-glycosides.