Atomic Absorption Spectroscopy Principle
Atomic absorption spectroscopy (AAS) and atomic emission spectroscopy (AES) principle are based on the absorption and emission of light by atoms in the gaseous state. It was first observed by Fraunhofer while studying dark lines in the solar spectrum. In 1955 Alan Walsh from Australia applied the principle and instrumentation of atomic absorption spectroscopy for the analysis of chemical elements. Many difficult and time-consuming instruments were replaced after the discovery of AAS.
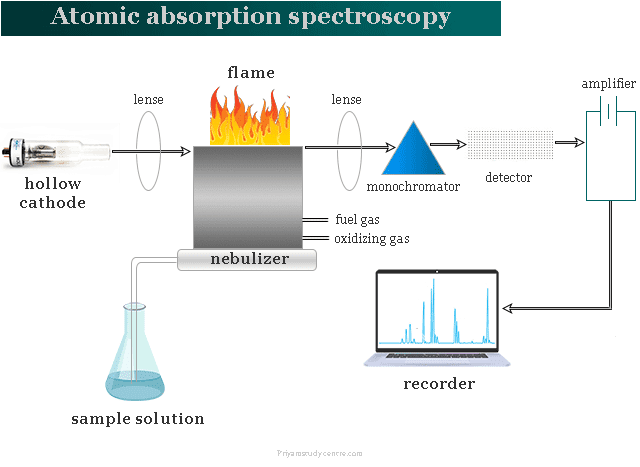
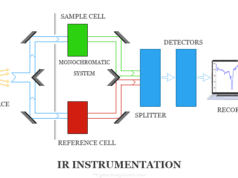
Atomization, hollow cathode lamp, monochromator, detector, and recorder are the main components in atomic absorption spectroscopy instrumentation. Elements with low excitation energy can be determined by flame emission while high excitation energy can be determined by atomic absorption spectroscopy.
Atoms absorb light at a definite wavelength depending on the nature of chemical elements.
Light at this wavelength has absorbed energy to excite another electronic state. The electronic transition is specific to a particular element. From the ground state of an atom is excited to a higher energy level by absorption of energy.
Atomic spectra are identified by sharp lines which can be distinguished from broadband spectra associated with molecules. Usually, lines arising from the ground state are almost important in atomic absorption spectroscopy. These are called resonance lines.
Atomic Absorption Spectroscopy Flame
We used fuel and oxidant to create an atomic absorption spectroscopy flame. Usually, natural gas, propane, butane, hydrogen, and acetylene are used as fuels to create a flame.
Air, oxygen, nitrous oxide, and a mixture of nitrous oxide and acetylene are used as an oxidant for flame creation in atomic absorption spectroscopy.
A list of various flames with maximum temperate is given below the table,
| Atomic absorption spectroscopy flame | |||
| Fuel | Air oxidant | Oxygen oxidant | Nitrous oxide oxidant |
| Hydrogen | 2100 °C | 2780 °C | – |
| Acetylene | 2200 °C | 3050 °C | 2955 °C |
| Propane | 1950 °C | 1950 °C | – |
We used low temperatures for metals like copper (Cu), lead (Pb), zinc (Zn), and cadmium (Cd). These metals are easily vaporized at low temperatures.
Some metals are not easily atomized or vaporized. Therefore, we need a high temperature for the vaporization of such metals. Such high temperatures can be attained by using an oxidant in the flame along with fuel gas in atomic absorption spectroscopy.
For example, we used oxyacetylene flame for the analysis of aluminum, titanium, and rare earth elements in an AAS instrument.
Atomic Absorption Spectroscopy Instrumentation
For instrumentation, flame, non-flame, and graphite furnace are available in atomic absorption instruments. Any atomic absorption spectroscopy instrumentation has the following types of components,
- Atomization
- Hollow cathode lamp
- Monochromator
- Detector
- Recorder
Atomization
Atomization can be carried out either by a flame or furnace. Heat energy is utilized in atomic absorption spectroscopy to convert metallic elements to atomic dissociated vapor. The temperature should be controlled very carefully for the conversion of atomic vapor. At too high temperatures, atoms can be ionized.
Fuel and oxidant gases are fed into a mixing chamber which passes through baffles to the burner. A ribbon flame is produced in the AAS instrument. The sample is aspirated through the air into the mixing chamber.
Hollow Cathode Lamp
We need a continuous source of radiation in the AAS instrument. The extreme narrowness of the absorption line in the sources causes problems. We used a hollow cathode glow discharge lamp to give sharp emission lines for a specific element in atomic absorption spectroscopy instrumentation.
The hollow cathode lamp has two electrodes, one is cup-shaped and made of a specific element. Radiation from the hollow cathode lamp should not be continuous due to spurious radiations. Therefore, we used a chopping wheel between the radiation or pulsed potential.
The metal which is used in the cathode is the same as that metal that we analyzed. The lamp is filled with noble gas at low pressure. The lamp forms a glow of emission from the hollow cathode.
Several types of hollow cathode lamps are available in our market. Such types of lamps are called multi-element hollow cathode Lamps. Such types of lamps facilitate for determination of samples without change of lamps each time. These types of lamps are widely used in atomic absorption spectroscopy instruments.
Monochromator
A monochromator is an optical device that transmits a narrow band of wavelengths of light or other radiation from a wider range of wavelengths. The atoms in the AAS instrument accept the energy of excitation and emit radiation.
A desired band of lines can be isolated with a monochromator by passing a narrow band. The spectra through a monochromator can be shown by a curve.
Detector
A detector can convert light coming from a monochromator to a simplified electrical signal. Generally, we used a photomultiplier tube as a detector in the atomic absorption spectroscopy instrument. A detector can be tuned to respond by a specific wavelength or frequency.
Recorder
The recorder can receive electrical signals from the detector to convert them into a readable response. In atomic absorption spectroscopy instrumentation, today we used a computer system with suitable software for recoding signals coming from the detector.
Application of Atomic Absorption Spectroscopy
Today, the atomic absorption spectroscopy technique is the most powerful tool in analytical chemistry, forensic science, environmental analysis, and food industries. It is popular for analysts due to several advantages.
- The most important advantage is the speed of analysis. It can analyze various samples within a day.
- Secondly, it is possible to determine all elements at trace concentration.
- Thirdly, it is not always essential to separate the element before analysis because AAS can be used to determine one element in presence of another.
- The atomic absorption spectroscopy principle or instrumentation can be used to analyze sixty-seven metals and several nonmetals such as phosphorus and boron.