What is sodium?
Sodium is a soft, low melting, silvery white alkali metal or chemical element of Group 1 or IA of the periodic table with the symbol Na and atomic number 11. It is extensively involved in our life process and many of its compounds are used from the early days of human civilization. It was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide (NaOH).
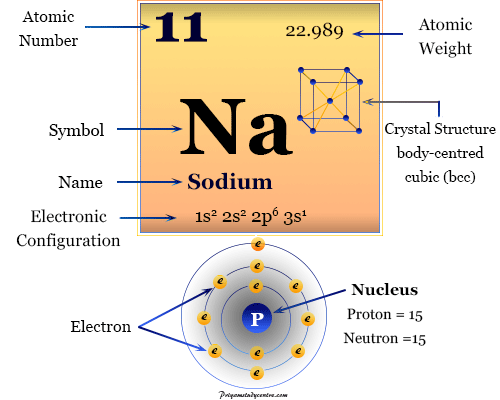
The electronic configuration of the alkali metal sodium is 1s2 2s2 2p6 3s1. Due to the presence of 3s1 outer electronic configuration, the alkali metal sodium occupies Group-1 or 1A on the periodic table.
Properties of sodium
The physical and chemical properties of the element are readily understood in terms of its simple outer electronic configuration. Some physical and chemical properties like melting point, boiling point, common oxidation number or state, and density are given below the table.
| Properties of sodium | |||
| Name | Sodium | ||
| Symbol | Na | ||
| Name derived from | The English word ‘soda’ | ||
| Atomic number | 11 | ||
| Discovery | Humphry Davy in 1807 | ||
| Electron per shell | 2-8-1 | ||
| Electronic configuration | 1s2 2s2 2p6 3s1 | ||
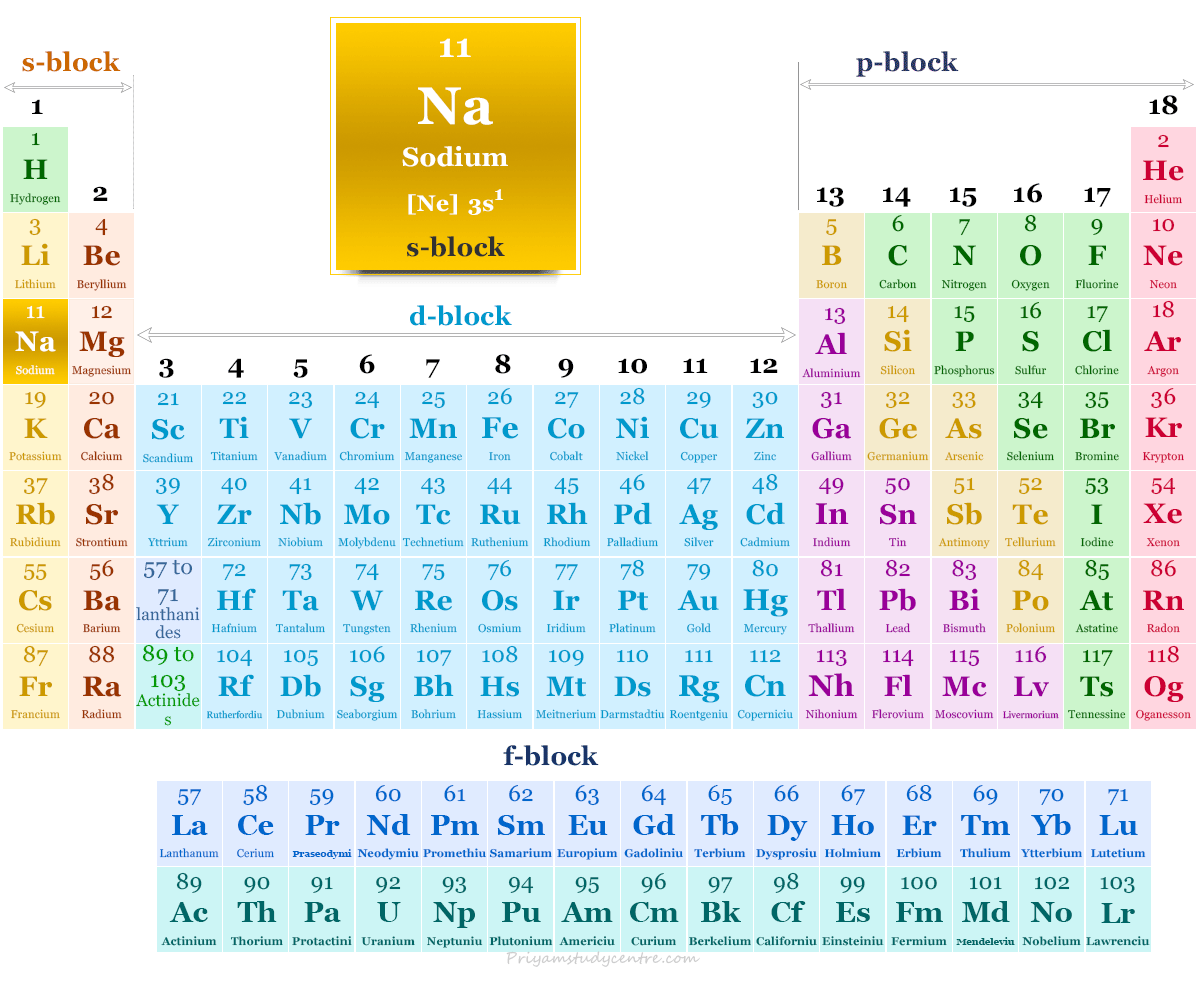
| Group | group-1 | ||
| Period | period-3 | ||
| CAS number | 7440-23-5 | ||
| Atomic weight | 22.989 | ||
| State at 20°C | Solid | ||
| Melting point | 97.79 °C, 208.03 °F | ||
| Boiling point | 882.94 °C, 1621.29 °F | ||
| Common isotope | 23Na | ||
| Density | 0.968 g/cm3 | ||
| Molar heat capacity | 28.230 J mol−1 K−1 | ||
| Electrical resistivity | 47.7 nΩ·m | ||
| Atomic radius | 186 pm | ||
| Covalent radius | 166±9 pm | ||
| Chemical properties | |||
| Oxidation number | +1 | ||
| Electronegativity | Pauling scale: 0.93 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 495.8 | 4562 | 6910 | |
Position of sodium on Periodic table
Where is sodium found?
Sodium is the seventh most abundant periodic table element and the fifth most abundant metal after aluminum, iron, calcium, and magnesium. It has found 22,700 ppm in the earth’s crust. It has occurred in many minerals like rock salt (NaCl), carbonate (trona), nitrate (saltpeter), borate (borax), etc.
The vast deposit of rock salt may be obtained by the evaporation of ancient seas. The Cheshire salt field in the United Kingdom occupies areas of (60 km × 24 km) and a nearly 400-meter thick layer. Similar deposits are found in Saskatchewan, Canada, and Carlsbad, New Mexico. Besides the rock salts, natural brines and oceanic waters contain large amounts of sodium chloride.
Sodium in living organisms
Plants and animal organism contains sodium compounds mainly NaCl. Na+ ion constitutes about 0.3 percent of human blood, 0.6 percent of bone, and 0.6 to 1.5 percent of body muscles.
Production Process
Sodium is made by the electrolysis of a fused mixture of NaCl and CaCl2. The temperature for electrolysis is considerably lower than the melting point of pure sodium chloride (803 °C). Therefore, the difficulties from the volatility of Na (boiling point 883 °C) are largely eliminated.
Under such conditions, the discharge potential of the Na+ ion is lower than that of the Ca+2 ion. It is preferentially deposited with 1 to 2 percent of calcium metal in a cylindrical steel cathode. Chlorine liberated by this production process at the central graphite electrode (anode) is collected through the nickel dome.
Facts about sodium
- All the alkali metals like lithium, sodium, potassium, and rubidium are soft, low melting, silvery-white, metals but cesium is a golden yellow colour.
- They are normally adopting a body-centered cubic crystal structure but at low temperature, lithium forms a hexagonal closed-packed arrangement.
- Only one electron in the 3s orbital of the metal atom takes part in metallic chemical bonding. It makes the metal soft and low-melting materials.
- The large difference between the first and second ionization energy of sodium suggests that the preferred oxidation state of the metal will be +1.
Chemical compounds
The first ionization energy of metal is more than compensated lattice energy suggesting that most of the compounds are formed by ionic bonding. Hence major chemistry of the element is dominated by ionic bonding. The reactivity of alkali metals towards water increases from lithium to cesium.
At 25 °C water reacts with lithium slowly, sodium reacts vigorously, potassium reacts with flame, and rubidium and cesium react with the explosion. It burns with air or oxygen to give Na2O2. Sodium reacts with carbon to form acetylides Na2C2. It forms a series of crystalline solid salts with different types of anions that are soluble in water.
Sodium hydride
It reacts directly react with hydrogen when heated to form an ionic hydride containing H− ion. All the alkali metal halides are colorless, water-soluble crystalline solids formed by the reaction of MOH or M2CO3 with appropriate HX.
Uses of Sodium
- More than half of the sodium produced every year is used for the production of Na/Pb alloy in the manufacture of lead tetraethyl (an antiknock compound). The production and manufacture of lead tetraethyl are likely to be decreased due to environmental pollution or lead posing.
- It is used as a reducing agent in the extraction of titanium and zirconium.
- A considerable amount of metal is consumed in the production of various types of sodium compounds like hydroxide (NaOH), peroxide (Na2O2), hydride (NaH), organosodium compounds, etc.
- Dispersion of sodium in various media like carbon and potassium carbonate is used as a chemical catalyst in various reactions of alkenes. These are used for the production of artificial rubber.
- The metal has a low melting point, low viscosity, and low neutron absorption cross-section with high heat capacity and thermal conductivity. Therefore, sodium is the most favorable material for heat exchange in the fast breeder nuclear power reactor.
NaCl uses
- The use of sodium chloride in the industry is simply numerous. It is the starting material for the production of NaOH, Na2CO3, Na2SO4, etc.
- About 50 percent of NaCl is used for the manufacture of NaOH and about 10 percent is used for making Na2CO3.
- It is widely used in food preservation.
- In certain winter counties, large quantities of NaCl are used to clear the snow on highways.
- Paper, rubber, water softening, and tanning industries are the chief consumers of NaCl.
Uses of NaOH
- Sodium hydroxide is largely used in the manufacture of different types of chemicals like alcohol or phenol, resorcinol, hypochlorite, phosphate, etc.
- A wide quantity of NaOH is used in the paper, pulp, and rayon industries. It is also used for the extraction of aluminum.
Sodium carbonate uses
- It is largely used in the glass industry, paper industry, in swimming pools for maintenance pH scale.
- Sodium carbonate is also used for the production of personal care products like detergents, soaps, and toothpaste.