Chromium Metal
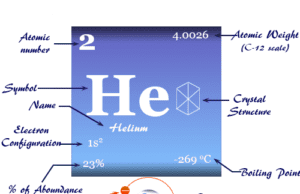
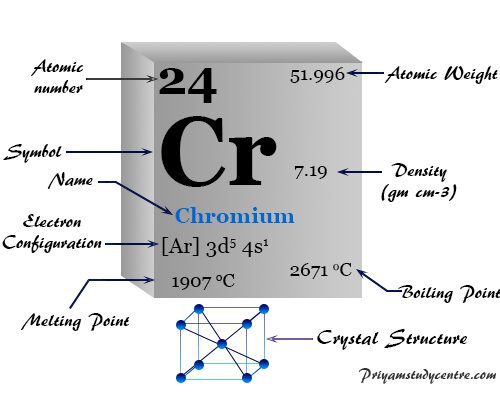
Chromium is a chemical element or hard, malleable, greyish-white shiny transition metal of Group 6 (VIB) of the periodic table with atomic number 24 and symbol Cr. It is widely used in making stainless steel and other alloys. It is readily detected by the borax bead test (green) and the yellow melt of chromates is formed during fusion with alkali or an oxidizing agent. The compounds of chromium chromates produce a deep blue colour with hydrogen peroxide in an acid solution. In 1797, chromium was discovered by French chemist Nicolas-Louis Vauquelin from Siberian mineral-like PbCrO4, and the name of the element was derived from the Greek word chroma means colour. Body-centered cubic crystal lattice, chromium has a valence shell electronic configuration [Ar] 3d5 4s1.
Properties of Chromium
The oxidation state vs free energy diagram shows that the stable oxidation number or state of chromium is +3. The melting point and density of chromium are higher than those of vanadium. It suggests less participation of d-electrons in metallic chemical bonding.
| Chromium | |||
| Symbol | Cr | ||
| Discovery | Nicholas Louis Vauquelin in 1798 | ||
| Name derived | The Greek word chroma means colour | ||
| Common isotope | 24Cr52 | ||
| Oxidation states | −4, −2, −1, 0, +1, +2, +3, +4, +5, +6 | ||
| CAS number | 7440-47-3 | ||
| Periodic Properties | |||
| Atomic number | 24 | ||
| Relative atomic mass | 51.996 | ||
| Electron per cell | 2, 8, 13, 1 | ||
| Electronic Configuration | [Ar] 3d5 4s1 | ||
| Block | d-block | ||
| Group | 6 | ||
| Period | 4 | ||
| Physical Properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1907 °C, 3465 °F, 2180 K | ||
| Boiling point | 2671 °C, 4840 °F, 2944 K | ||
| Molar heat capacity | 23.35 J mol−1 K−1 | ||
| Crystal structure | body-centered cubic (bcc) | ||
| Density | 7.15 g/cm3 | ||
| Electrical resistivity | 125 nΩ m | ||
| Atomic Properties | |||
| Atomic radius (non-bonded) | 2.06 Å | ||
| Covalent radius | 1.30 Å | ||
| Electronegativity | 1.66 (Pauling scale) | ||
| Electron affinity | 64.259 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 652.87 | 1590.63 | 2987.19 | |
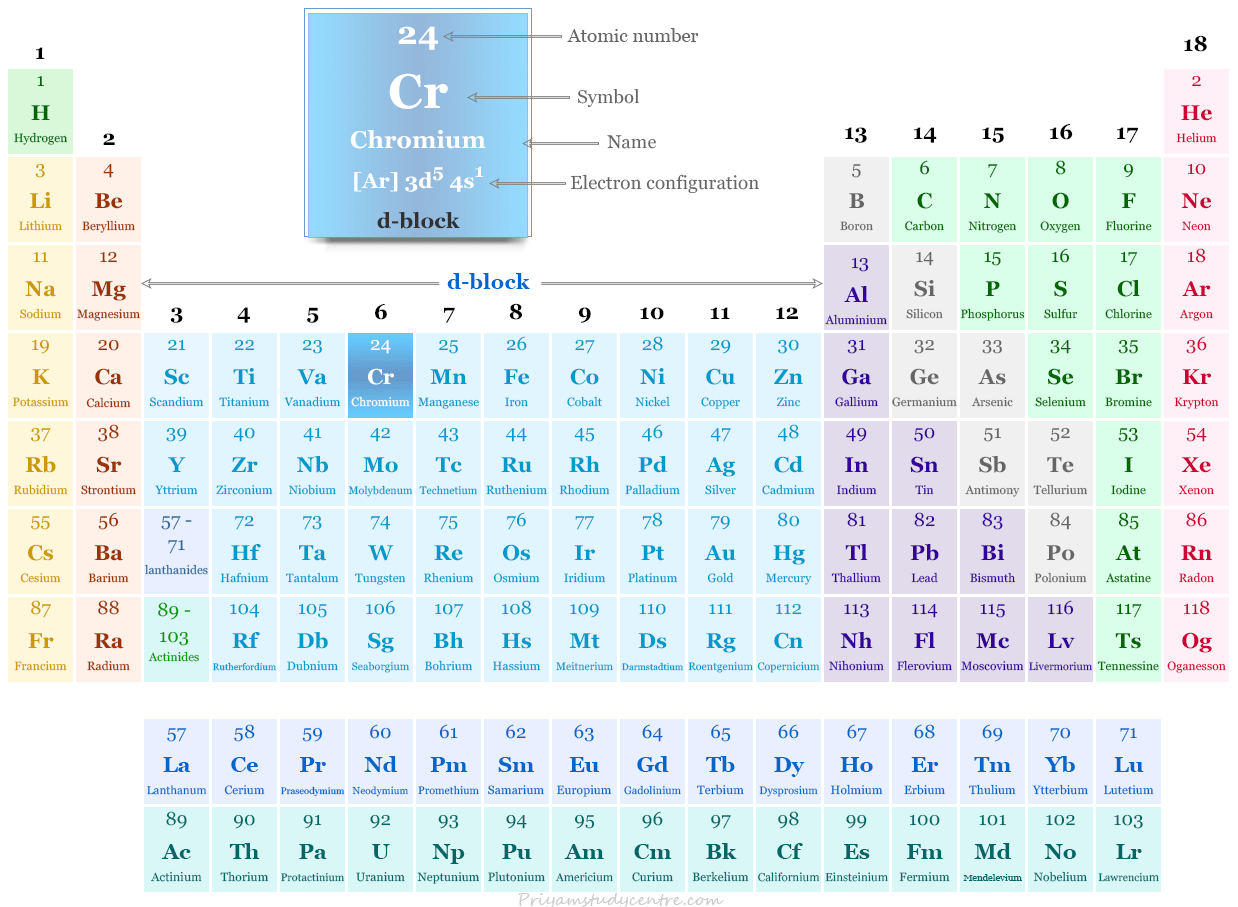
Chromium in the Periodic Table
It is placed in group 6 and period 4 of the periodic table. Chromium is a transition metal that lies between vanadium and manganese.
Where is Chromium Found?
It is the 21st most abundant chemical element in the earth’s crust (0.04 percent by weight). Chromium occurs mainly as chromite (FeCr2O4), found in South Africa, India, the Philippines, Turkey, and Russian countries.
Crocoite (PbCrO4) and chrome ochre (Cr2O3) are the other minerals of the metal found in the earth’s environment.
In gemstones, some chromium ion (Cr+3) ions are substituted by aluminum (Al+3) ions. The presence of Cr+3 and Cr+4 ions in the earth’s environment strongly depends on the pH scale and oxidative properties of the soil of location.
Isotopes of Cr
Naturally, the chemical element, chromium contains four stable isotopes like 52Cr (83.76 percent), 53Cr (9.55 percent), 50Cr (4.31 percent), and 54Cr (2.38 percent). It has also nineteen radioactive isotopes produced from different types of radioactive decay or nuclear reaction.
Extraction of Chromium
Greyish-white shiny metal, chromium is extracted from chromite (Cr2O3).
- Powdered chromite is heated (1000 °C to 1300 °C) with sodium carbonate and quicklime in a revelatory furnace in the presence of excess air.
- Metallic chromium is oxidized to form sodium chromite and iron is converted to F2O3.
- The sodium chromite is leached out with water and converted to sodium dichromate by adding concentrated sulfuric acid.
- The produced dichromate is reduced to Cr2O3 by heating with carbon.
- Metallic chromium is obtained during the reduction of Cr2O3 by aluminum powdered through the Thermite process.
Cr2O3 + 2 Al → Al2O3 + Cr - The pure form of the element may be obtained by reducing Cr (III) chloride with metallic calcium in the melting solution of CaCl2 or BaCl2.
Interesting Facts About Chromium
Chromium is the fourth transition metal after scandium, titanium, and vanadium in the periodic table with electron configuration [Ar] 3d5 4s1.
The electron configuration of Cr violates the Aufbau principle. Therefore, it can recording of 3d orbital to form a new electronic configuration to gain extra chemical stability by exchanging energy.
It dissolves slowly in dilute hydrochloric acid and sulfuric acid with evolving hydrogen. However, in concentrated nitric acid, it forms an impervious oxide layer.
In chemistry, the common and dominant oxidation state of chromium is +3 and +6. It also forms organometallic compounds in +1, +2, +4, and +5 oxidation states.
Chemical Compounds
Chromium Oxide
The metallic chromium offers a fair number of Cr (II) compounds including oxides and dihalides. The oxide CrO is a black powder formed by exposing chromium amalgam to air. It is not well characterized.
The common and most stable oxidation state of the metal is Cr (III). Therefore, it forms the most stable trivalent cation in water solution and a series of substituted inert metallic complexes. The oxide, Cr2O3 is formed by burning chromium in oxygen or heating ammonium dichromate.
Chromium (VI) Oxide
The principal chromium oxide in the +VI state is CrO3. Chromium trioxide is formed when adding concentrated sulfuric acid to a concentrated aqueous solution of potassium dichromate.
The oxide is highly acidic and soluble in water solution to form H2CrO4 (pH scale = 1), HCrO4− (pH scale > 1), and Cr2O7−2. Therefore, they are powerful oxidizing agents that oxidize most materials like paper, sugar, oxalic acid, and alcohol.
Glacial acetic acid is not oxidized by it and the glacial acetic acid solution of chromium trioxide uses as an oxidizing agent in chemistry.
Chromium Halides
All four dihalides (CrF2, CrCl2, CrBr2, and CrI2) are obtained by reducing CrX3 with hydrogen. They can be also obtained by reducing metal and gaseous HX or iodine at 700 °C to 1000 °C.
The Cr (II) halides are reducing in nature, they can liberate hydrogen from water in the absence of an oxidant.
All three halides CrCl3 (red-violet), CrBr3 (dark green), and CrI3 (dark green) are prepared by a direct combination of the metal with halides like chlorine, bromine, and iodine at various temperature regions.
Compounds of Cr (III)
Green colour, CrF3 is prepared by direct combination of the metal with fluorine but it may be prepared better by heating CrCl3 with HF at 500 °C.
The sulfide compounds can be made by direct reaction of chromium with sulfur or anhydrous CrCl3 with hydrogen sulfide.
Compounds of Cr (VI)
Compounds of Cr (VI) are very impotent and limited. The principal species in the +6 state are CrO3, unstable CrF6. It also forms oxohalides CrOX4 (X = F, Cl), CrO3X2, and CrO3X−, the oxoanions and peroxo molecule.
Chromyl Chloride
Chromyl chloride is the most important oxohalide of Cr (VI). It is formed when ionic chloride is heated with potassium dichromate and concentrated H2SO4.
It is a violent oxidizing agent inflammable in phosphorus, sulfur, alcohol, etc. Chromyl chloride forms another violent oxidizing agent in reaction with nitrogen pentoxide (N2O5).
Sodium or potassium chromates and dichromate are also two important chemicals of chromium widely used as an oxidizing agent and analysis of redox reactions.
Uses of Chromium
In Making Stainless Steel
About 85 percent of chromium is used in making stainless steel and other alloys in metallurgy. However, the reminder part is used in different types of chemical, refractory, and industries. It makes steel tough and corrosion-resistant.
- Chromium steel (1.2 to 2 percent Cr) is mostly used in cutting tools, oil tubing, automobile trim, armor plates, and ball bearings making.
- Steels with higher chromium contact (17 to 18 percent) and 7 percent of nickel have superior corrosion resistance. Therefore, they are used in jet engines, gas turbines, and common structural supplements.
- Cr-vanadium steel (1 percent Cr and 0.15 percent of vanadium) and Cr-tungsten steel (3.8 percent Cr and 14 20 percent W) are used mostly for making springs, shafts, axles, and different types of high-speed tools.
- Nichrome (60 percent of Ni, 14 percent of Cr, 25 percent of iron, and 0.2-1 percent carbon) is used in resistance coils for electrical heating due to its high melting point, high electrical resistance, and low oxidation properties.
Other Uses
- Several chromium compounds are used generally as a chemical catalyst for the production of hydrocarbon.
- It is used as a pigment due to its bright green, yellow, red, and orange colours.
- Chromium salt is used for the preservation of wood due to the toxic nature of Cr (IV) salts.
- About 90 percent of leather is tanned by using chrome alum and Cr (III) sulfate compounds.
- It is also used in chromium plating which does not damage by sulfur compounds present in the earth’s atmosphere.