Oxidation and Reduction Reaction
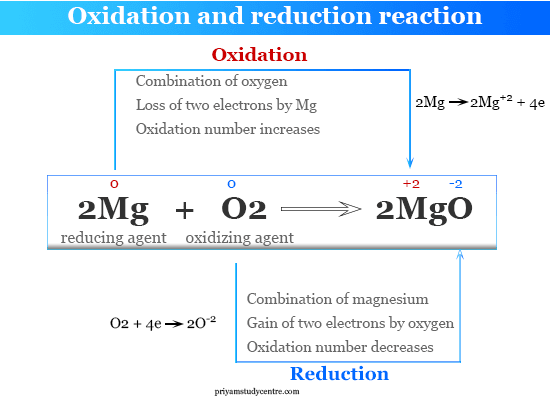
Oxidation reduction reaction or Oxidizing and reducing agents are always found to go hand in hand during redox reactions. Whenever one element or molecule is oxidized, another element or molecule must be simultaneously reduced. Combination and removal of oxygen or hydrogen, loss or gain of electrons, and increases or decreases in oxidation number of participation atoms or ions are used to define the oxidation-reduction process or concept in a chemical reaction. We know magnesium burns in oxygen to produce crystalline solid magnesium oxide. The same metal when reacts with chlorine, produces magnesium chloride. In both chemical reactions, magnesium is considered to be oxidized and oxygen or chlorine is considered to be reduced. In this example, magnesium metal is the reducing agent, and oxygen or chlorine is the oxidizing agent.
What is Oxidation in Chemistry?
- Oxidation is classically defined as the combination of oxygen or any other electronegative element with another element or the removal of hydrogen or electropositive element from chemical compounds.
- According to the electronic concept, oxidation is a process that results in the loss of one or more electrons by participating atoms or ions.
- According to the oxidation number concept, oxidation may be defined as a process where the oxidation number of participating atoms increases.
What is Reduction in Chemistry?
- The reduction is classically defined as the combination of hydrogen or any other electropositive element with another element or the removal of oxygen or electronegative element from chemical compounds.
- According to the electronic concept, reduction is a process that results in the gain of one or more electrons by participating atoms or ions.
- According to the oxidation number concept, reduction may be defined as a process where the oxidation number of participating atoms decreases.
Oxidizing and Reducing Agent
- An oxidizing agent is one that gains electrons and reduces to a lower valency.
- A reducing agent is one that loses electrons and oxidizes to a higher valency.
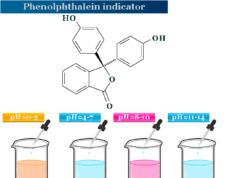
Oxidizing and Reducing Agents Examples
In an acid medium, potassium permanganate oxidized iron (II) to iron (III) state. In this reaction, the permanganate ion is an oxidizing agent, and the iron (II) ion or ferrous ion is a reducing agent.
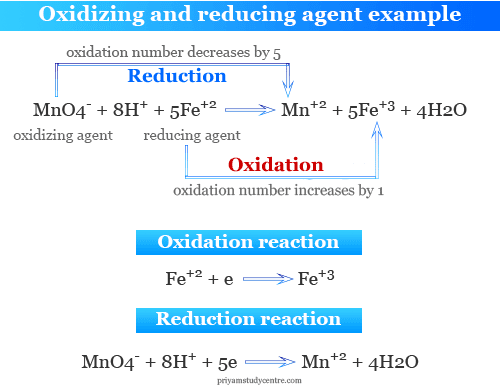
The second example is related to the oxidation of potassium iodide by potassium dichromate in a dilute sulfuric acid medium.
In this reaction, chromium ion changes their oxidation state or number from +6 to +3. Therefore, dichromate is an oxidizing agent. Similarly, iodine changes its oxidation state from −1 to 0. Therefore, potassium iodide is a reducing agent.
| Common oxidizing agents | ||
| Gaseous | Liquid | Solid |
| oxygen | nitric acid | pottasium permaganate |
| ozone | sulfuric acid | potassium dichromate |
| fluorine, chlorine | hydrogen peroxide | manganese dioxide |
| nitrogen dioxide | liquid bromine | iodine |
| Common reducing agents | ||
| Gaseous | Liquid | Solid |
| hydrogen | nitrous acid | carbon |
| sulfur dioxide | hydrobromic acid | sodium |
| hydrogen sulfide | hydroiodic acid | aluminum |
| carbon monoxide | sulfurous acid | stannous chloride |
Electronic Concept of Oxidation and Reduction
The electronic configuration of elements gives the quantitative relations between classical and electronic concepts of oxidation and reduction reaction.
For example, the formation of crystalline solid magnesium oxide by an oxidation reduction reaction.
- Each magnesium atom loses two electrons to form a magnesium ion (cation).
- A neutral oxygen atom gains two electrons to form an oxygen ion (anion).
These two ions formed a closed-packed magnesium oxide crystal lattice by chemical bonding.
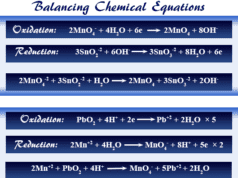
| Oxidation reduction reaction | |
| 2 Mg + O2 → 2 MgO | |
| Oxidation | Reduction |
| 2 Mg → 2 Mg+2 + 4e | O2 + 4e → 2O−2 |
| 2 Mg+2 + 2 O−2 → 2 MgO | |
The above balancing chemical equation shows that oxidation and reduction go hand in hand with the formation of stable magnesium oxide.
- Above reaction, magnesium lost two valence orbital electrons to form a stable noble gas electronic configuration.
- Similarly, oxygen has gained these two electrons to form a stable neon electronic configuration.
Oxidation Reduction Reaction Example
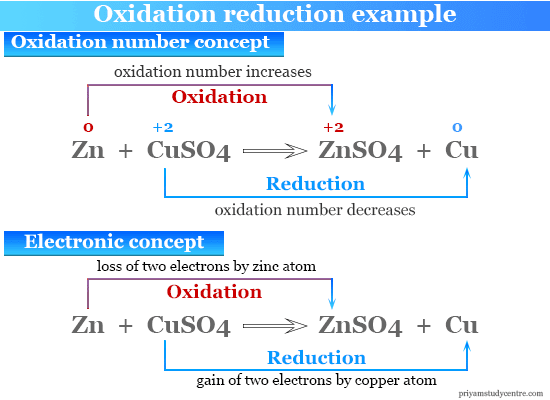
Oxidation Number Concept
Above reaction given in the picture oxidation number of zinc increases from 0 to +2. Therefore, it is called oxidation according to the definition.
Similarly, the oxidation number of copper decreases from 0 to −2. Therefore, it is called a reduction reaction according to the definition. Oxidation and reduction always go hand in hand during this redox reaction.
Electronic Concept of Oxidation and Reduction
According to the electronic concept, the zinc atom loses two electrons to form a Zn+2 ion by the oxidation process. Similarly, copper atoms gain these two electrons to form Cu+2 ions through the reduction process.
Oxidation Reaction Examples
Combination of Oxygen
All the hydrocarbon like methane, ethane, and propane readily burns in excess air or oxygen to form carbon dioxide and water. Controlled oxidation gives various products.
CH3-CH3 + 3 [O] → 2 CO2 + 3 H2O
For example, oxidation in the presence of boric acid produces a mixture of a secondary alcohol.
An oxidizing agent such as potassium permanganate really oxidizes tertiary hydrogen atoms to form the hydroxyl group.
(CH3)3CH + [O] → (CH3)3COH
Combination of Electronegative Element
Group-17 elements in the periodic table stable on a negative oxidation state. The oxidation power of group-17 elements decreases from fluorine to iodine.
The electronegative element chlorine oxidizes the colorless FeCl2 solution to form red FeCl3.
2 FeCl2 + Cl2 ⇆ 2 FeCl3
Removal of Hydrogen
When manganese dioxide is heated with concentrated hydrochloric acid, it produces greenish-red chlorine gas. Hydrogen can be removed from hydrochloric acid to form chlorine gas.
4 HCl + MnO2 ⇆ MnCl2 + Cl2 + H2O
Oxidation of hydrogen sulfide to sulfur in the presence of nitric acid. In this oxidation reduction reaction, hydrogen can remove from the hydrogen sulfide molecule.
H2S + 2 HNO3 ⇆ S↓ + 2 NO2 + 2 H2O
Removal of Electropositive Elements
Hydrogen peroxide oxidizes hexacyanoferrate (II) to hexacyanoferrate (III) in the presence of an acid solution.
2 [Fe(CN)6]4− + H2O2 + 2 H+ → 2 [Fe(CN)6]4− + 2 H2O
Example of Reduction Reaction
Addition of Hydrogen
Alkenes are rapidly added hydrogen atom or hydrogenated under pressure in the presence of chemical catalyst like platinum or palladium surface.
C2H4 + H2 → C2H6
Reduction of monocarboxylic acid in organic chemistry. The reduction of monocarboxylic acid depends on the nature of the reducing agents, heat, and catalyst used.
Monocarboxylic acid like acetic acid heated with hydrogen iodide and red phosphorus under pressure produced alkenes. It also heats with hydrogen under pressure at a specific heat in the presence of a nickel catalyst producing the same product.
RCO2H + 3 H2 → RCH3 + 2 H2O.
Addition of Electropositive Elements
Hydrogen peroxide reduces hexacyanoferrate (III) to hexacyanoferrate (II) in the acidic solution.
| 2K3[Fe(CN)6] + 2KOH + H2O2 ⇆ 2K4[Fe(CN)6] + 2H2O + O2 |
In the above oxidation reduction reaction, potassium ferricyanide combines with the electro-positive element potassium to produce potassium ferrocyanide.
Removal of Oxygen
When hydrogen passes through heated black color CuO, oxygen is removed from CuO to form red copper.
CuO + H2 ⇆ Cu +H2O
Removal of Electronegative Elements
When SO2 passes through a red ferric sulfate solution, it turns into a greenish ferrous sulfate and sulfuric acid.
Fe2(SO4)3 + 2 SO2 + H2O ⇆ 2 FeSO4 + 2 H2SO4
In the above reaction, electronegative sulfate is removed from ferric sulfate, and ferric sulfate is reduced to form ferrous sulfate in oxidation reduction or redox reaction.