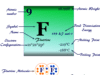
Nitrogen Element Symbol
Nitrogen is a nonmetallic chemical element of group 15 of the periodic table with the symbol N or a gas molecule with the molecular formula N2. The group members nitrogen and phosphorus are essential elements of the living system while the dinitrogen molecule is a colourless, odorless, tasteless gas that uses mainly to create an inert atmosphere in meteorological production. Dinitrogen is the most plentiful substance in the earth’s atmosphere which is present in air 78% by volume and 75% by weight but the abundance of nitrogen in rocks and soil of the earth’s environment is remarkably low (about 19 ppm) comparable to gallium, niobium, and lithium. In the nitrogen cycle, nitrogen can continuously interchange between the atmosphere and the biosphere through the nitrogen fixation process. A large-scale production or generation of nitrogen gas is carried out by burning hydrocarbon in the air.
Only nitrogenous minerals like potassium nitrate (saltpeter) and sodium nitrate (chile saltpeter) are isolated via nitric acid by the action of nitrifying or nitrogen fixation bacteria.
Who Discovered Nitrogen?
Carl Wilhelm Scheele and Henry Cavendish had independently discovered and isolated nitrogen but the credit for discovery was given to Scottish physician Daniel Rutherford in 1772. Rutherford was given this credit because his work was published first in a science journal.
The name nitrogen was given by French chemist Jean-Antoine-Claude Chaptal in 1790. He may derive the name from the Greek word ‘nitron’ and ‘genes’ meaning nitre forming.
Where is Nitrogen Found?
Major deposits of nitrogen compounds like nitrate are found in Bolivia, Italy, Spain, Russia, and some regions of India. It is an essential component of all living systems (plants and animals) Therefore, the major human interference occurs through the artificial fixation of N2 by fertilizers to meet the increasing demand for foods in our growing human family.
Nitrogen in the Periodic Table
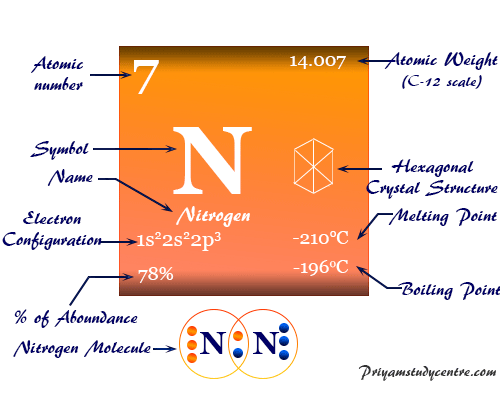
The electronic configuration of nitrogen is 1s2 2s2 2p3. Therefore, it is placed in group 15 and period 2 of the periodic table.
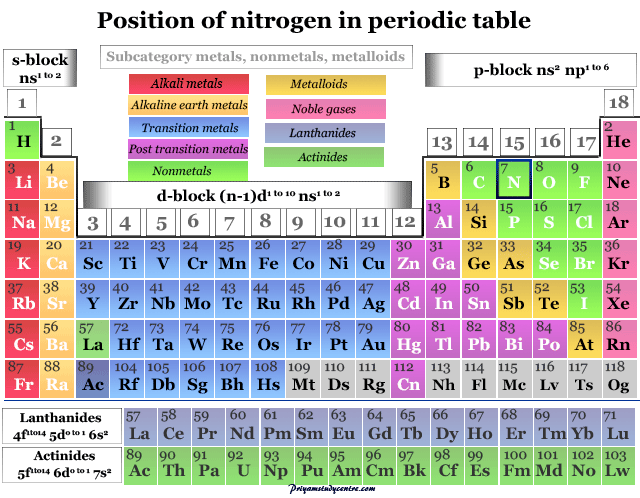
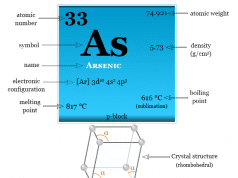
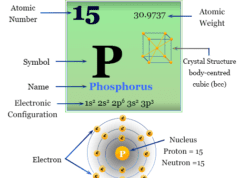
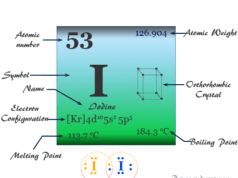
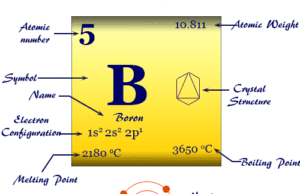
Nitrogen is a p-block element placed along with the group members phosphorus, arsenic, antimony, and bismuth. The group members nitrogen and phosphorus are essential constituents of the living system. However, the heavier members in the group, particularly arsenic are extremely toxic to human beings.
Stable Isotopes
In learning chemistry, naturally occurring nitrogen exists mainly in two stable isotopes, 14N and 15N. The relative abundance of 14N:15N ≈ 272:1. The radioactive isotope, 7N15 may be prepared or separated by an exchange reaction or by thermal diffusion.
15NO (g) + 14NO3− (aq) → 14NO + 15NO3− (aq)
In 1919, a British physicist, Ernest Rutherford first introduced artificial facts of radioactive nuclear reaction by the bombardment of the alpha ray on 14N isotope to form 17O and proton particles.
Properties of Nitrogen
The elemental form of nitrogen (symbol N) is an essential constituent of the living system while the molecular form is a colorless gaseous substance with the chemical formula N2. This colorless, odorless, and tasteless gas is almost insoluble in water. The liquid form of dinitrogen is a colorless gas that freezes to a white solid. Dinitrogen is incombustible and a non-supporter of combustion.
| Nitrogen |
|||
| Symbol | N | ||
| Discovery | Daniel Rutherford in 1772 | ||
| Name derived from | The Greek word nitron and genes mean nitre forming | ||
| Allotropes | Dinitrogen (N2) | ||
| Common isotope | 7N14 | ||
| Crystal structure | Hexagonal crystal lattice | ||
| Periodic properties |
|||
| Atomic number | 7 | ||
| Electron per shell | 2, 5 | ||
| Atomic weight | 14.007 | ||
| Electronic configuration | [He] 2s2 2p3 | ||
| Group | 15 | ||
| Period | 2 | ||
| Block | p-block | ||
| Physical properties |
|||
| State at 20 °C | Gas | ||
| Melting point | −210 °C, −346 °F, 63.2 K | ||
| Boiling Point | −195.79 °C, −320.43 °F, 77.35 K | ||
| Critical temperature | 126.21 K | ||
| Density | 0.001145 g cm−3 | ||
| Chemical properties |
|||
| Atomic radius (non-bonded) | 1.55 Å | ||
| Covalent radius | 0.71 Å | ||
| Oxidation number or states | 5, 4, 3, 2, -3 | ||
| Ionization energy (kJ mol−1) | 1st | 2nd | 3rd |
| 1402.33 | 2856.09 | 5300.47 | |
| Electron affinity | Unknown | ||
| Electronegativity | 3.04 (Pauling scale) | ||
| Molar heat capacity |
29.124 J mol−1 K−1 | ||
| CAS number | 7727-37-9 | ||
Chemical properties
The outer quantum shell electronic configuration of nitrogen is 2s22p3. Therefore, it contains two paired s-electrons and three unpaired p-electrons. The electronic configuration suggests that the element is closer to the next noble gas than the preceding noble gas.
Oxidation number
Assuming the +5 cationic configurations by losing all the five outer electrons is just impossible. Therefore, most of the chemical compounds of nitrogen are covalent compounds with an oxidation state of +3 or +5. The oxidation number of the element covers a wide range from −3 (in NH3) to +5 (HNO3). Due to the non-availability of energetically vacant d-orbitals, it has no scope for valency expansion.
The chemistry of nitrogen describes that the atom may attain the next noble gas electronic configuration by gaining three electrons to form an N−3 ion (oxidation number = −3). However, such electron attachment involves a high positive enthalpy change.
The formation of nitride ion (N−3) requires 2130 kJ mol−1 energy. Therefore, it occurs only in nitride salts of strongly electropositive metals like lithium, beryllium, magnesium, calcium, etc.
Chemical Reactivity
The molecular form of the element or dinitrogen is generally unreactive at room temperature due to the following reasons,
- Dinitrogen has high N≡N bond energy.
- The molecular orbital description of dinitrogen is 1σg1 2σu2 1πu4 3σg2. Therefore, it shows a large difference between the HOMO (3σg) and LUMO (2πu).
- Symmetrical electron distribution also makes the dinitrogen molecule nonpolar.
For the above reason, the N2 molecule can react slowly at room temperature with Li and at elevated temperatures with Be, Mg, Ca, Sr, Ba, Al, B, Si, and Ge to form nitrides.
3 Mg + N2 → Mg3N2
Nitrogen combines with hydrogen at 550 °C and 220 atmospheric pressure in the presence of an iron catalyst to form ammonia.
N2 + 3H2 → 2 NH3 + 22.4 kcal
It also combines with red hot carbon to form cyanogen and oxygen at 3000 °C to form nitric oxide (NO).
N2 + O2 → 2 NO
Calcium carbide reacts with N2 at 800 °C to form calcium cyanamide. The brown-colored mixture of calcium cyanimide and carbon is known as nitrolim. It is used in making fertilizer.
CaC2 + N2 → CaNCN + C
N2 may absorbed by certain transition metal complexes to form coordination compounds of N2.
[Ru(NH3)5H2O]+2 + N2 → [Ru(NH3)5N2]+2 + H2O
[CoH3(PPh3)3] → CoH(PPh3)3N2 + H2
Reactive atomic nitrogen can be obtained when dinitrogen is passed at low pressure through an electric discharge tube. The formation of such active species is accompanied by a yellow afterglow which persists several seconds after the discharge is discontinued.
Nitrogen Production
Production From Air
Industrially or commercially large amounts of dinitrogen are obtained during the isolation of oxygen by fractional distillation of liquid air. The N2 molecule boils off before oxygen because its boiling point is lower (N2: −195.8 °C; O2: −183.1 °C). The product usually contains a fraction of argon.
The dinitrogen molecule can also be produced on a large scale by burning carbon or hydrocarbon in the air. The produced carbon dioxide and water are separated from the gas mixture.
Laboratory Preparation of Nitrogen
Nitrogen may be obtained in the laboratory by a variety of chemical reactions. Such production of nitrogen in laboratories has little utility except for academic purposes. Some of these production processes are given below,
N2 can be prepared in the laboratory by heating ammonium nitrite (NH4NO2). Some NO and HNO3 are formed by this process. Therefore, these must be removed by absorbents like potassium dichromate in the presence of sulfuric acid.
However, ammonium nitrite is a very unstable chemical compound and thermal decomposition takes place with an explosion. Therefore, we use a concentrated solution containing sodium nitrite and ammonium chloride for the production of N2 gas. In the first step, when heating gently, ammonium chloride reacts with sodium nitrite to form ammonium nitrite and sodium chloride.
NaNO2 + NH4Cl → NH4NO2 + NaCl
Therefore, ammonium nitrite produced from the above reaction may decompose in the second step to form dinitrogen and water.
NH4NO2 → N2 + 2 H2O
Production of nitrogen in the laboratory may also carried out by heating ammonium dichromate or sodium azide.
(NH4)2Cr2O7 → N2 + Cr2O3 + 4 H2O
2 NaN3 → 2 Na + N2
Other Laboratory Production
Dinitrogen can be also produced in the laboratory by heating aqueous ammonia and chlorine or bromine gas. Ammonia must be in excess during the reaction with chlorine, otherwise explosive NCl3 is formed.
8 NH3 + 3 Br2 → N2 + 6 NH4Br
8 NH3 + 3 Cl2 → N2 + 6 NH4Cl
The high-temperature reaction of ammonia with cupric oxide.
3 CuO + 2 NH3 → 3 Cu + N2 + 3 H2O
N2 can be obtained when passing ammonia into the suspension of bleaching powder.
3 Ca(OCl)Cl + 2NH3 → 3 CaCl2 + 3 H2O + N2
An effervescence of nitrogen gas is produced when sodium hypobromite reacts with urea. During such chemical reactions, urea decomposes by hypobromite to form N2 and carbon dioxide gas.
CO(NH2)2 + 3 NaOBr → N2 + CO2 + 2 H2O + 3 NaBr
Uses of Nitrogen
Nitrogen is an essential element of the living system because it is an impotent constituent of proteins. Dietary protein is almost an exclusive source of elemental nitrogen to the body. Therefore, nitrogen balance truly represents the protein utilization and its loss from the body. It can also be uses in the following chemical processes:
- Gaseous dinitrogen is an unreactive chemical species at room temperature. Therefore, it is largely used to provide an inert atmosphere in metallurgy.
- Nitrogen gas is also used in various chemical industries such as the iron and steel industry for welding, soldering, brazing, etc.
- The inert dinitrogen gas is extensively used in the petrochemical industry to create an inert environment for preventing explosions.
- A large scale of dinitrogen is used in the chemical industry for the manufacture of ammonia, calcium cyanamide, nitric acid, nylon, dyes, and many explosives.
- Dinitrogen is a useful chemical for cryogenic research.
- Dinitrogen is used as an inhibitor for fire or explosions. Therefore, it is used as fire extinguisher.
Uses of Liquid Nitrogen
Liquid nitrogen is an inert cryogenic fluid or a useful refrigerant with a temperature of − 196 °C. Freezing in such substances is the fastest freezing method due to its extremely low temperature. Therefore, the liquid form of such element can be used for cooling, chilling, and preserving foods, medical substances, biological specimens, etc.
- The liquid nitrogen is used in low-temperature machining.
- In grinding rubbers and rubber-like substances, we also used dinitrogen.
- Liquid nitrogen is also used for the preservation of biological specimens.
- In medicine, liquid nitrogen is used as a refrigerant for rapid freezing to preserve blood, bone marrow, tissue, bacteria, and semen.
Nitrogen Cycle
Nitrogen is an essential element for the growth of plant and animal life present in nature. It continuously interchanges between the atmosphere and the biosphere. To balance the nitrogen cycle in nature, it interchanges from the atmosphere to biosphere or biosphere to atmosphere by the following major steps,
- The action of nitrogen fixation bacteria
- Artificial fixation of dinitrogen
- Lighting in the upper atmosphere
- Death and decay of plants and animals
- Burning of wood, coal, and petroleum
- Drainage of surface water
Nitrogen Fixation by Bacteria
The conversion of free atmospheric N2 gas into a useful nitrogenous compound is called fixation of nitrogen. About 60 percent of N2 is input into the soil by the action of nitrifying bacteria (Rhizobium, Anabaena, Nostoc, Azotobactor, and Clostridium pastorium). These nitrifying bacteria directly convert dinitrogen into nitrates or ammonium salts.
Rhizobium is the most important bacteria in this category. It lives symbiotically in the nodules or roots of certain plants like Leguminosae, peas, beans, etc.
The nitrogen-fixing enzyme nitrogenase in fixed N2 into the soil. The fixation is subsequently greater than the artificial fixing through the Haber process (150 million tonnes vs 120 million tonnes).
Industrial Fixation
Industrial fixation is used to grow nitrogen into the soil for the production of large amounts of food for the ever-increasing population of the world. The main procedure involves the Haber process for the manufacture of ammonia which converts nitric acid and other fertilizers.
Other Processes to Fix Nitrogen
Lighting in the upper atmosphere leads to the formation of NO and NO2, which are carried by nitric acid rain to the soil. In higher temperatures during lighting, N2 in the air combines with O2 to form NO.
N2 + O2 → 2 NO
The nitric oxide obtained from the above reaction further reacts with oxygen to form nitrogen dioxide.
2 NO + O2 → 2 NO2
NO2 reacts with water during rain to form nitric acid and come into the soil. It reacts with alkalis present in soil to form soluble nitrates and is absorbed by plants as a nutrient.
2 NO2 + H2O → HNO2 + HNO3
The death or decay of plants and animals returns most of the nitrogen to the soil. The combustion of wood, coal, and petroleum also releases a small amount of NO2 into the atmosphere.
The drainage of surface water carries some nitrogen gas into the sea which uses to support the common marine life.
Frequently Asked Questions
What is nitrogen?
Nitrogen is a nonmetallic chemical element of group 15 of the periodic table with the symbol N or a gas molecule with the molecular formula N2. Elemental nitrogen is an essential component of living matter while dinitrogen molecule is a colourless, odorless, tasteless gas that uses commonly to create an inert atmosphere in meteorological production.
Why nitrogen is important?
Nonmetal nitrogen is a key component of life and an essential nutrient for both animals and plants. The molecular form dinitrogen is the most plentiful substance in the earth’s atmosphere which is present in the air 78% by volume and 75% by weight.
What fixes nitrogen?
Dinitrogen commonly fixes to the biosphere by the action of nitrogen fixation bacteria, artificial processes, or lighting in the upper atmosphere. About 60 percent of N2 is input into the soil by the action of nitrifying bacteria (Rhizobium, Anabaena, Nostoc, Azotobactor, and Clostridium pastorium).
Why is nitrogen fixation important?
Nitrogen is an essential component for the growth of plants and animals. Therefore, nitrogen fixation is important because molecular dinitrogen is an inert gas that cannot be directly used by plants and animals. Molecular dinitrogen converts to reactive inorganic nitrogen compounds through nitrogen fixation and is used for the growth of plants and animals.
What is nitrogen used for?
Nitrogen is an essential component of living organisms (plants and animals) but molecular dinitrogen gas plays an important role in making the inert atmosphere in metallurgy. We also used liquid nitrogen to create low temperatures for the preservation of biological specimens and medical substances such as blood, bone marrow, tissue, bacteria, semen, etc.