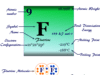
Oxygen Element
Oxygen, atomic number 8, symbol O, and molecular formula O2 is the most abundant chemical element of the Group-16 or chalcogen family in the periodic table. Molecular oxygen is the third chemical after sulfuric acid and nitrogen that uses in the industry in various production processes. Nonmetallic oxygen has properties to react with almost all other periodic table elements except halogens, noble gases, and some of the platinum metals. Such facts about elemental oxygen suggest that it makes up 47 percent of the earth’s crust in the form of gaseous, liquid, and solid oxides and oxoacids. Oxygen gas is essential to the living body (plants and animals) to perform various biological activities. Oxygen gas occurs in the natural environment about 21 percent by volume in air, and 86 percent by weight of liquid water in oceans.
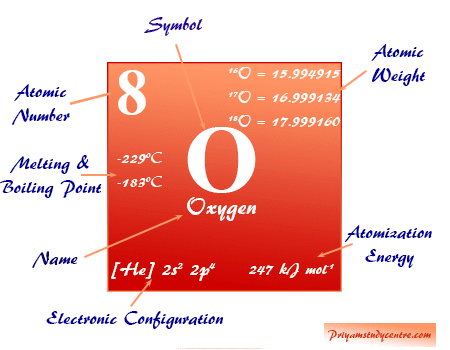
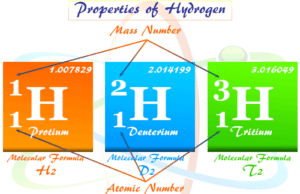
Gaseous oxygen occurs in two allotropic forms dioxygen and ozone with the chemical formula O2 and O3. The elemental oxygen atom also forms an isotopic mixture of 16O, 17O, and 18O isotopes.
Isotopes of Oxygen and Their Uses
Oxygen element has three known stable isotopes that are represented 16O, 17O, and 18O. Two isotopes, 17O and 18O can prepared by fractional distillation or electrolysis of water molecules or thermal diffusion of oxygen gas.
| Main isotopes | Relative atomic mass | Abundance |
| 16O | 15.994915 | 99.763% |
| 17O | 16.999134 | 0.0380% |
| 18O | 17.999160 | 0.205% |
Elemental oxygen isotope 18O is used as the tracer in chemical kinetics and mechanistic studies. Similarly, the isotope 17O (mass number = 17) is used in NMR spectrum analysis.
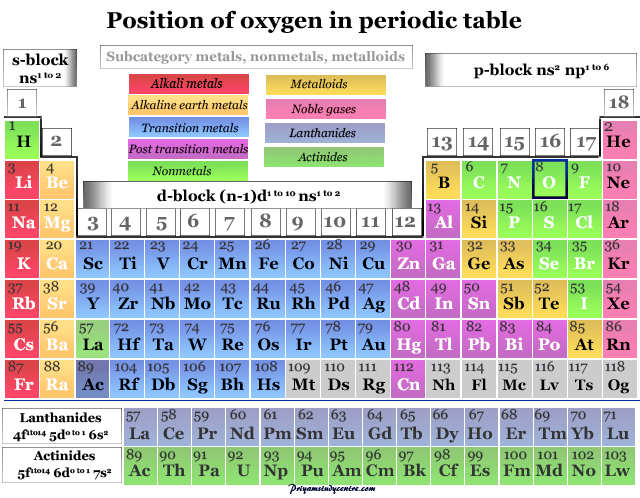
Oxygen on the Periodic Table
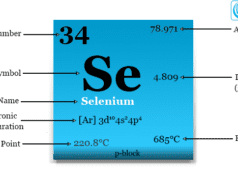
The nonmetallic chemical element oxygen is placed in group 16 of the periodic table. Therefore, it is a p-block element placed with sulfur, selenium, tellurium, and polonium. The position of oxygen element in the periodic table is given below the picture,
Properties of Oxygen
Dioxygen is a powerful oxidizing agent comparable to that of acidified dichromate solutions. However, the reaction is not carried out in ordinary conditions. Therefore, a solution of Fe (II) slowly oxidized in air at room temperature even though the process is thermodynamically favorable.
½ O2 + 2 H+ + 2 e− → H2O (E° = +1.23 V)
The rates of oxidation by oxygen may also increase remarkably when transition metal ions are used as a catalyst. The formation of oxide ions in the gas phase is an endothermic process. It is compensated by the high lattice energy of many metal oxides.
| Oxygen |
|||
| Symbol | O | ||
| Discovery | Joseph Priestley and independently by Carl Wilhelm Scheele in 1774 | ||
| Name derived from | The Greek word oxy genes, meaning acid-forming | ||
| Allotropes | O2 and O3 | ||
| Common isotope | 8O16 | ||
| Crystal structure | Cubic crystal lattice | ||
| Periodic properties |
|||
| Atomic number | 8 | ||
| Electron per shell | 2, 6 | ||
| Atomic weight | 15.999 | ||
| Electronic configuration | 1s2 2s2 2p4 | ||
| Group | 16 | ||
| Period | 2 | ||
| Block | p-block | ||
| Physical properties |
|||
| State at 20 °C | Gas | ||
| Melting point | −218.79 °C, −361.82 °F, 54.36 K | ||
| Boiling Point | −182.96 °C, −297.33 °F, 90.19 K | ||
| Density | 0.001308 g cm−3 | ||
| Chemical properties |
|||
| Atomic radius (non-bonded) | 1.52 Å | ||
| Covalent radius | 0.64 Å | ||
| Oxidation number or states | −1, −2 | ||
| Ionization energy (kJ mol−1) | 1st | 2nd | 3rd |
| 1313.94 | 3388.67 | 5300.47 | |
| Electron affinity | 140.976 kJ mol−1 | ||
| Electronegativity | 3.44 (Pauling scale) | ||
| Molar heat capacity |
29.378 J mol−1 K−1 | ||
| CAS number | 7782-44-7 | ||
The bond dissociation energy of O2 is high (496 kJ mol−1). Therefore, reactions involving such dissociation require high energy of activation.
Physical Properties
Dioxygen (molar mass = 16 gm/mol) is a colourless, odourless, tasteless gas that occurs in two allotropic forms dioxygen (molecular weight = 32 g/mol) and ozone gas (molecular weight = 48 g/mol).
The colorless, orderless dioxygen molecule is paramagnetic in nature with two unpaired electrons. Dioxygen is fairly soluble in water but highly soluble in organic solvents like acetone and benzene and forms weak charge-transfer complexes.
Dioxygen is a pale blue liquid but forms a blue solid crystal lattice after converting to a solid. In liquid or solid form, a single photon collides with other molecules and excites both. Absorption of electromagnetic spectrum radiation in the red region to the green visible region gives the observed blue color of solid or liquid oxygen.
Chemical Properties
Oxygen (meting point = − 229 °C and boiling point = − 183 °C) has very high ionization energy among the group 16 chemical elements. Therefore, it has a very low metallic character and is commonly known as a nonmetal.
With the increasing atomic number in group 16, the resistance of the elements decreases. Therefore, oxygen and sulfur are insulators, selenium and tellurium are semiconductors, but polonium is a metal that conducts electrical energy.
Bonding in Oxygen Molecule
The electronic configuration of oxygen element is 1s2 2s2 2p4. Therefore, the element is two electrons short of the next noble gas configuration and achieves these two bonding electrons by gaining two electrons from electropositive elements. However, it may also achieved by making two single covalent bond or one double bond with the same or other elements.
In an O2 molecule, the two oxygen atoms share two electrons with each other to form two covalent bonds (one sigma and one pi bond). The sigma bond in the oxygen molecule is formed by the axial overlap of 2p atomic orbitals and the pi bond is formed by side on overlap of 2p atomic orbitals.
Oxygen has very high electronegativity and electron affinity in favor of the formation of crystalline solid ionic oxides. Therefore, when it reacts with magnesium, it forms crystalline magnesium oxide.
Mg → Mg+2 + 2 e−
O + 2 e− → O−
Mg+2 + O−2 → MgO (Ionic crystal)
Elemental oxygen has properties for the formation of ionic compounds with alkali metals (lithium, sodium, potassium) and alkaline earth metals (beryllium, magnesium, calcium).
Oxidation Number of Oxygen
The elemental oxygen generally shows a −2 oxidation number or state in a chemical compound due to the 2s2 2p4 outer electronic configuration. However, in peroxide (hydrogen peroxide) and superoxides, the oxidation number of oxygen is −1 and −½ respectively.
In F2O, the oxidation number of oxygen element is +2 because fluorine is more electronegative than oxygen and shows a −1 oxidation number.
Interesting Facts About Oxygen
In learning chemistry, the outermost quantum shell of oxygen consists of the 2s2 2p4 electronic configuration. Therefore, oxygen element alone in the group16 or chalcogen family that does not possess d-orbital. Such a fact suggests that it only shows valency 2.
However, due to the presence of vacant d-orbitals other Group 16 elements (sulfur, selenium, tellurium, and polonium) can show 2, 4, and 6 valances for chemical bonding purposes.
Molecular Orbital Diagram of Oxygen
The valence shell electronic configuration of the oxygen atom is 2s2 2px2 2py1 2pz1 with six electrons. Therefore, in the O2 molecule, we have a total of twelve electrons to accommodate in the molecular orbitals.
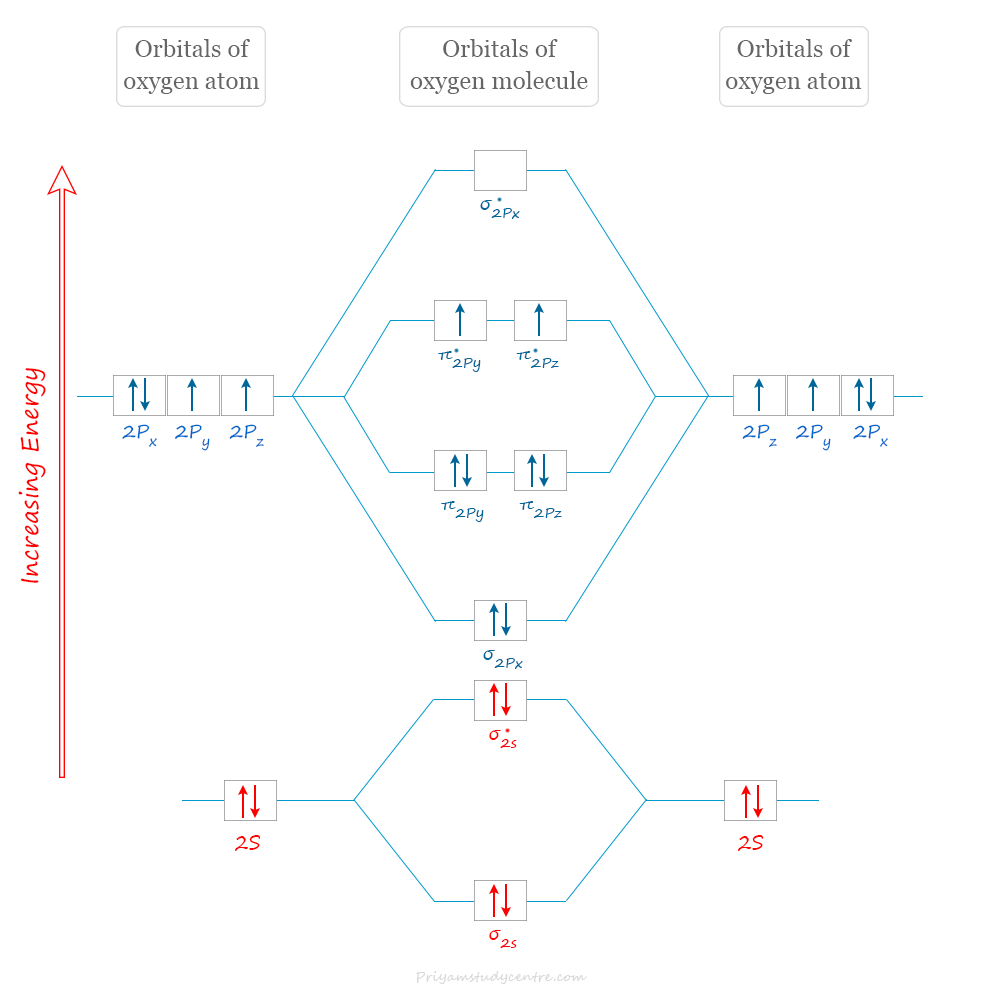
The molecular orbital electronic configuration of the O2 molecule:
σ2s2, σ∗2s2, σ2pz2, π2px2 = π2py2, π∗2px1 = π∗2py1
From the above electronic configuration, the bond order of the O2 molecule = ½(8 − 4) = 2. Therefore, the O2 molecule contains a double bond where one is a sigma bond and the other is a pi bond.
In the ground state, the highest occupied molecular diagram of the O2 molecule shows the two electrons (parallel spin) in two pi-antibonding orbitals. It is said to be the triplet state.
However, the next higher state is one in which the electrons are spin-paired in pi-antibonding molecular orbitals. These states are termed singlet states. Normally, a triplet to singlet transition is forbidden. Therefore, gaseous O2 is a colourless chemical compound.
Production Process
Oxygen element or gas molecule is obtained industrially by fractional distillation of liquid air. However, in biochemistry, oxygen is produced as a byproduct during photosynthesis where plants use carbon dioxide and water for the production of glucose and oxygen in the presence of sunlight.
6 CO2 + 6 H2O + photons → C6H12O6 (glucose) + 6 O2
Therefore, during photosynthesis, carbon dioxide and water are used up by plants and many other microorganisms for the production of glucose (carbohydrates) and oxygen.
In the laboratory, it may be prepared from different chemical compounds like potassium chlorate and potassium permanganate, etc. It can also be produced by the catalytic decomposition of hydrogen peroxide over the platinum catalyst in nickel foil.
2 H2O2 → 2 H2O + O2
Alkaline Water Electrolysis
Electrolysis of 30 percent potassium hydroxide (KOH) solution with nickel electrodes produced oxygen in the anode and hydrogen in the cathode. The electrochemical reactions occurring at the cathode and anode are:
- Cathode (reduction): 2 H2O → H2 + 2 OH−
- Anode (oxidation): 2 OH− → ½ O2 + H2O
- Overall reaction: H2O → H2 + ½ O2
Potassium hydroxide is preferred over sodium hydroxide due to the higher conductivity of potassium hydroxide solution.
Preparation of Oxygen from Potassium Chlorate
The thermal decomposition of potassium chlorate produces O2 at 400° to 500 °C.
2 KClO3 → 2 KCl + O2
However, if we use manganese dioxide as a chemical catalyst, the reaction occurs at 150 °C. Such a process may also produce 3 percent of chlorine dioxide (ClO2).
The thermal decomposition of pure potassium permanganate in a vacuum at specific heat also gives a very pure O2 molecule.
2 KMnO4 → K2MnO4 + MnO2 + O2
Singlet Oxygen
Singlet oxygen has been prepared in several ways:
- It can be prepared by irradiation of O2 in the presence of a sensitizer like fluorescein, methylene blue, etc.
- Singlet oxygen can also be produced during decomposition of ozonides or hydrogen peroxide.
H2O2 + Cl2 → 2 H+ + 2 Cl− + O2∗
We can reprent siglent O2 in fist transition state by O2∗. It has a sufficient lifetime to enter into chemical and biological reactions.
What is Oxygen Used For?
Oxygen is the third chemical in order of use in the industry after sulfuric acid and nitrogen. Therefore, nearly 100 million tonnes of O2 is consumed annually throughout the world.
- It is largely used in metallurgy like steel making in blast furnaces and Bessemer converters.
- It is also used in direct oxidation in many chemical processes.
- The colorless, odorless, tasteless O2 gas molecule is used for making synthesis gas in chemistry.
- In organic chemistry, it is also used for oxidizing organic hydrocarbons like methane, ethane, ethylene, acetylene, etc.
- It is an oxidizer for the fuels in rocket propulsion.
Uses in Our Animal or Plant
The processes that all living organisms perform to maintain their life are called life processes. Oxygen is an essential chemical element for the life process of all animal or plant bodies.
Oxygen in Animal Body
In the animal body, it is carried out by two metalloproteins hemoglobin and myoglobin. Besides hemoglobin and myoglobin, there are also two other dioxygen transport proteins hemocyanins and hemerythrin. Hemerythrin is found in marine invertebrates.
An adult human contains about 5 liters of blood. Each milliliter of blood contains 5000 million blood cells and each cell contains 0.25 million hemoglobin molecules. The red blood cells have a life span of 100 to 120 days. Therefore, one percent of hemoglobin molecules are replaced daily.
Oxygen Transport in Blood
Hemoglobin is essential for oxygen transport in our bodies because one molecule of hemoglobin can bind four molecules of oxygen. Myoglobin is engaged in the storage of O2 in muscle tissues and is used when necessary.
O2 molecules in our body are used in the biosynthesis of many compounds in the metabolic chain. Oxygen may also convert some lipid-soluble molecules to water-soluble ones for excretion.
Oxygen in Plant Body
In plant leaves, gaseous exchange takes place by diffusion of oxygen molecules through stomata into the cells of the leaf. The direction of diffusing mainly depends on the environmental conditions and requirements of plants.
- During the daytime, when photosynthesis occurs, carbon dioxide is rapidly taken up by plants while oxygen release is the major event. Therefore, during the daytime, O2 molecules diffuse in leaves and CO2 molecules diffuse out from leaves.
- On the other hand during the night, the elimination of carbon dioxide from plants is a major event. Therefore, during the daytime, CO2 molecules diffuse in leaves and O2 molecules diffuse out from leaves.