What are Redox Reactions?
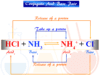
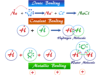
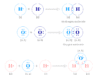
Redox reactions are oxidation-reduction reactions where reacting substances change their oxidation number or state to form products. In other words, a redox reaction is a chemical reaction where one or more electrons are transferred between two reacting substances (atoms or ions) participating in it. Redox reactions have wide industrial applications such as electricity generation, purification or extraction of metals like zinc, aluminum, chromium, and copper by electrolysis, and combustion of fuel in the fuel cell. Indirect redox reactions take place in the electrochemical cell for the conversion of chemical energy into electrical energy by the transfer of electrons. The electrolytic cell is another example where electrical energy is converted to chemical energy by the transfer of electrons. In a redox reaction, an oxidant is reduced and a reductant is simultaneously oxidized. Redox reaction based on oxidation number and electronic concept in chemistry is given below the picture,
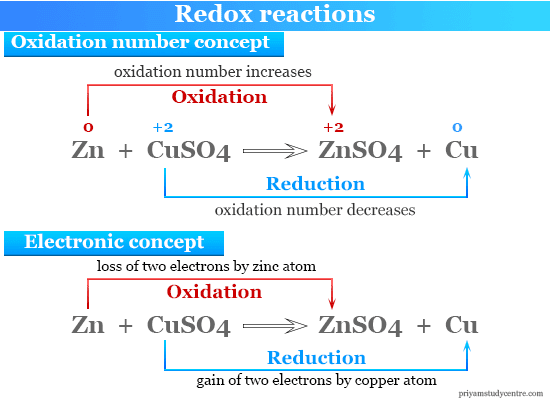
Oxidation and Reduction
Oxidation and reduction are always found to go hand in hand during a redox reaction.
- Oxidation may be defined as a process where the oxidation number of reacting substances increases.
2 S + O2 → SO2
CH4 + 2 O2 → CO2 + 2 H2O - Similarly, reduction may be defined as a process where the oxidation number of reacting substances decreases.
CH2=CH2 + H2 → CH3−CH3
2 FeCl3 + H2 → 2 FeCl2 + 2 HCl
Oxidizing and Reducing Agents in Redox Reactions
- Oxidizing Agent: The substance that can bring about the oxidation of other substances is called an oxidizing agent. In other words, a reagent that can increase the oxidation number of an element in the redox process is called an oxidizing agent.
- Reducing Agent: The substance that can bring about the reduction of other substances is called a reducing agent. In other words, a reagent that can decrease the oxidation number of an element or ion in the redox process is called a reducing agent.
Identify the Oxidizing Agent and Reducing Agent
3 Mg + N2 → Mg3N2
In the above redox process, the oxidation number of magnesium increses from 0 to +2, and nitrogen decreases from 0 to −3. Therefore, magnesium acts as a reducing agent, and nitrogen acts as an oxidizing agent.
2 FeCl3 + H2S → 2 FeCl2 + S + 2 HCl
In the above redox process, the oxidation number of iron decreases from +3 to +2, and sulfur increses from −2 to 0. Therefore, ferric chloride (FeCl3) acts as an oxidizing agent, and hydrogen sulfide (H2S) acts as a reducing agent.
Examples of Oxidizing and Reducing Agents
We can see various redox processes or oxidizing and reducing agents in our daily lives. The most common and usable oxidizing and reducing agents are listed below in the table,
| Oxidizing Agent | Reducing Agent |
| Group 17 elements (fluorine, chlorine, bromine, etc) | All metals (sodium, zinc, iron, aluminum) and several non-metals (carbon, hydrogen, sulfur, phosphorus, etc) |
| The compound where metal is in its highest possible oxidation state or number (KMnO4, K2Cr2O7, HClO4, etc) | Metallic hydrides (NaH, LiH, CaH2, etc) |
| Mineral acids (hydrochloric acid, nitric acid, sulfuric acid) | Hydracids (HBr, HI, H2S) |
| Compounds where an element in a higher oxidation state (FeCl3, SnCl4, etc) | Compounds contain an element in the lower oxidation state (FeCl2, SnCl2, etc) |
| Bleaching agents (ozone, hydrogen peroxide, chlorine dioxide, etc) | Organic compounds (formic acid, acetic acid, etc) |
The compounds in the above table contain the most common oxidizing agents that we use widely in our daily lives.
Identifying Oxidizing Agent and Reducing Agent in Redox Reactions
- When an element is in its highest possible oxidation state or number in a compound, it can function as an oxidizing agent. For example, KMnO4 (oxidation state of Mn = +7), K2Cr2O7 (oxidation state of Cr = +6), HNO3 (oxidation state of N = +5), H2SO4 (oxidation state of S = +6), and HClO4 (oxidation state of Cl = +7).
- If an element is in its possible lower oxidation state or number in a compound, it can be functioning as a reducing agent. For example, H2S (oxidation state of S = −2), FeSO4 (oxidation state of Fe = +2), and SnCl2 (oxidation state of Sn = +2).
- A compound is functioning as an oxidizing agent when a highly electronegative element is in its highest oxidation state.
- A compound functions as a reducing agent when a highly electronegative element is in its lowest oxidation state.
Types of Redox Reactions
Redox reactions are generally the following types,
- Decomposition reaction
- Combination reaction
- Displacement reaction
- Disproportionation reaction
Examples of Decomposition Reaction
The chemical reaction where one reactant can be broken down into two or more products is called a decomposition reaction. Decomposition can be represented by,
AB → A + B
Breakdown of hydrogen peroxide or water into hydrogen and oxygen are examples of redox reactions where decomposition can occur.
H2O2 → H2 + O2
2H2O → 2H2 + O2
All the decomposition reactions are not redox reactions. For example, decomposing calcium carbonate into calcium oxide and carbon dioxide is not a redox reaction.
CaCO3 → CaO + CO2
Examples of Combination Reaction
In a combination reaction, a metal can be reacted with non-metal to form an ionic crystalline solid. For example, when magnesium metal burns in oxygen to produce magnesium oxide.
2 Mg + O2 → 2 MgO
In the above reaction, two electrons can be transferred between the two reacting substances like magnesium and oxygen. Other examples of combination reactions are,
H2 + Cl2 → 2 HCl
C + O2 → CO2
4 Fe+ 3 O2 → 2 Fe2O3
Examples of Displacement Reaction
A displacement reaction is a type of chemical reaction where one or more atoms can be displaced by another atom. For example, the electron is transferred from the zinc atom to the copper atom in the electrochemical cell.
CuSO4 + Zn → Cu + ZnSO4
Examples of Disproportionation Reaction
A disproportionation reaction is a type of redox reaction where one element of a reactant in a particular oxidation state can be simultaneously oxidized and reduced to form two products.
For example, the chemical reaction between phosphorus and sodium hydroxide. Here phosphorus can simultaneously be oxidized and reduced.
P4 + 3 NaOH + 3 H2O → 3 NaH2PO2 + PH3
Examples of Redox Reactions
We can find various types of redox reactions in our daily life. For example, photosynthesis is an example of redox reactions where green plants convert carbon dioxide and water into carbohydrates.
6 CO2 + 6 H2O → C6H12O6 + 6 O2
In photosynthesis, carbon dioxide is reduced to carbohydrates while the water gets oxidized to oxygen. The energy required to carry out such a reaction is obtained from sunlight. It is the source of energy for all living systems.
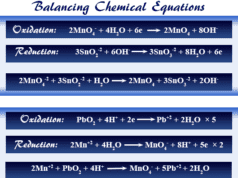
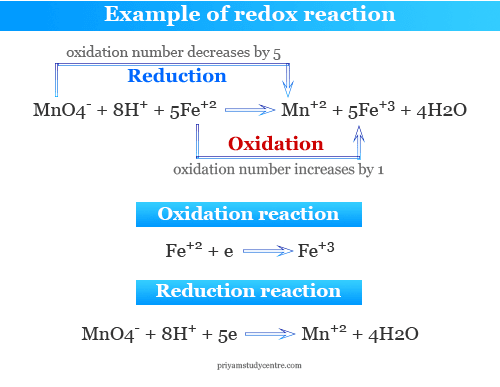
Oxidation of Iron by Potassium Permanganate
Oxidation of ferrous ion to ferric ion by acidic potassium permanganate is an example of a redox reaction where oxidation and reduction occur simultaneously. The half-reaction is given below the picture,
Reaction of Potassium Dichromate with Potassium Iodide
Oxidation of potassium iodide by potassium dichromate in a dilute sulfuric acid medium is another example of a redox reaction. Here chromium (+6) is reduced to chromium (+3) and iodine is oxidized to form an elementary iodine molecule.
Oxidation reaction:
2 I− → I2 + 2 e−
Reduction reaction:
Cr2O7−2 + 14 H+ + 6 e− → 2 Cr+3 + 7 H2O
Redox reaction:
Cr2O7−2 + 14 H+ + 6 I− → 2 Cr+3 + 3 I2 + 7 H2O
Redox Electrode Examples
- When oxidation (loss of electrons) reactions take place on the redox electrode, the chemical potential of the electrode is called oxidation potential.
- When reduction (gain of electrons) takes place on a redox electrode is called reduction potential
If the oxidation potential of an electrode = x volt, then its reduction potential = − x volt.
Two half-cell chemical reactions of ferric ion and ceric ion in the presence of dilute sulfuric acid are examples of redox electrodes. Two electrode potentials in balancing chemical equations can be given below in the table,
| Redox electrode examples | |
| Ce+4 + Fe+2 ⇄ Ce+3 + Fe+3 | |
| Half cell reaction | Electrode potential |
| Ce+4 + e− ⇄ Ce+3 | + 1.44 volt |
| Fe+2 ⇄ Fe+3 + e− | − 0.77 volt |
Applications of Redox Reaction
Redox reactions have wide industrial applications, it is used for constructing electrochemical cells such as voltaic or galvanic cells and electrolytic cells in our daily life.
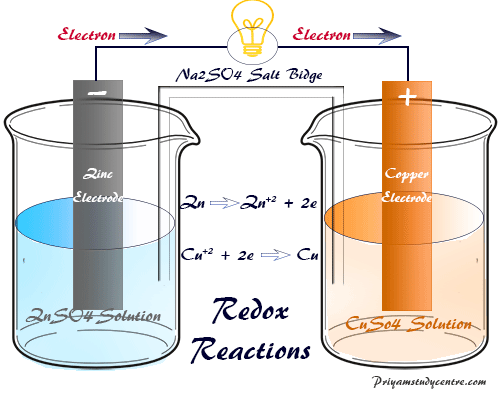
Redox Reaction in Electrochemistry
In the voltaic or galvanic cell, redox reactions take place to convert chemical energy into electrical energy by the electron transfer process. Therefore, the voltaic or galvanic cell is the electrochemical device in which energy is produced due to the indirect redox reaction and converted electricity for mankind.
The galvanic cell and Voltaic cell are examples of electrochemical cells that were named by the scientists, Luigi Galvani, and Alessandro Volta.
Application of Redox Reaction in Metallurgy
An electrolytic cell is an electrochemical device where a non-spontaneous redox reaction takes place by an external source of electricity. The electrolysis process occurs in the electrolytic cells.
The very useful application of electrolysis is found in commercial electroplating and purification of metals. For the electroplating of copper, an electroplating reaction is carried out by dipping copper into the suspension of an aqueous copper sulfate solution.
The oxidation and reduction reaction at the two electrodes can be represented,
At cathode: Cu+2 + 2 e− → Cu
At anode: Cu → Cu+2 + 2 e−
Cell reaction: Cu+2 + Cu → Cu + Cu+2
Electrolytic refining can be used for refining many metals such as copper, zinc, nickel, silver, gold, etc. In electrolytic refining, a thick block of impure metal is used in the anode and a thin strip of pure metal is used in the cathode. A solution of metal salts that we want to refine is used as an electrolyte.
- When an electric current is passed, metal ions from the electrolyte are reduced to form pure metal and deposited on the cathode.
- An equivalent amount of pure metal from the anode gets oxidized to metal ions and goes into the electrolyte solution. From there it goes to the cathode and is deposited.
The redox process is repeated until the whole of the metal ion from an impure block is dissolved and deposited on the cathode.
Application of Fuel Cell
A fuel cell is an electrochemical cell where chemical energy available from the fuel like methane, hydrogen, solid oxide, and alkaline is converted into electrical energy by redox reactions.
At the cathodes of the cell, oxygen is dissolved to form OH− ions. The cell has low voltage but is capable of supplying current for long periods.
Such cells provide power at a comparatively low cost and low maintenance. The efficiency depends particularly on the surface of the electrodes. It serves as the chemical catalyst for the attainment of chemical equilibrium.
The electric polarization is due to the adoption and poisoning of the redox electrode. It reduces cell performance considerably. The density of the electrolyte and the temperature have a great influence on the potential of redox reactions.
Real-life Examples of Redox Reaction
- Photosynthesis is a common example of a biological reaction that is carried out by the redex process.
- Redox reactions based on electrolysis are used widely in real life for the production of many chemicals such as caustic soda, caustic potash, chlorine, etc.
- Industrial production cleaning and bleaching agents can be carried out by the oxidation process.
- Nitric acid, a component of many fertilizers can be produced by the oxidation of ammonia.
- The redox process can be used in electroplating. For example, it is used for the production of gold-plated jewelry.
- Many metals separated from their ores and refined from impure metal can be carried out by redox reactions.
Frequently Asked Questions (FAQs)
Is every chemical reaction a redox reaction?
No, not every chemical reaction is carried out through the redox process. For example, double decomposition of salts and acid-base neutralization are considered to be non-redox reactions.
- Acid-Base Neutralization:
HNO3 + NaOH = H2O + NaNO3
2 HCl + CaCO3 = H2O + CaCl2 + CO2 - Decomposition reactions:
NH4Cl = HCl + NH3
CaCO3 = CO2 + CaO
What are the oxidizing and reducing agents in redox reactions?
In redox reactions, an oxidizing agent is a substance that gains one or more electrons and a reducing agent is a substance that loses one or more electrons.
- According to the oxidation number concept, a reagent that can increase the oxidation number of an element or ion is called an oxidizing agent in the redox process.
- Similarly, a reagent that can decrese the oxidation number of an element or ion is called a reducing agent in the redox process.
How to balancing redox reactions?
We can balance redox reactions in two ways ion electron method and the oxidation number method.
- The ion-electron method is based on the loss and gain of electrons during the redox process.
- The oxidation number method is based on the change in the oxidation number of two elements from reactant to product.
How to identify redox reactions?
Oxidation and reduction always go hand in hand in a redox reaction and are identified by changes in oxidation numbers.
- Oxidation is a process in which an increase in oxidation number occurs.
- Similarly, reduction is a process in which a decrese in oxidation number occurs.
Therefore, a chemical reaction where the oxidation number one element increases and the other element decreases from the reactant side to the product side is a redox reaction.