Spectroscopy
Spectroscopy Instruments
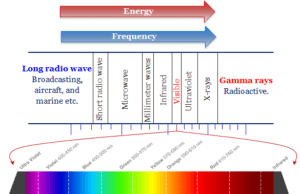
Spectroscopy in learning chemistry or physics is an analysis technique for analyzing the emission or absorption spectrum of electromagnetic radiation from matter (atom, ion, and molecule) by a suitable equipment or instrument (prism). Therefore, spectroscopy instruments are the most important tools or devices for spectroscopic analysis of the atomic spectra of periodic table elements and molecular spectra of inorganic and organic compounds. In this part of online learning chemistry, we study electromagnetic and hydrogen spectra, wavelengths, atomic emission and absorption spectroscopy instrumentation, UV, mass spectrum, infrared radiation (IR), electron spin resonance (ESR), etc.