Pressure Measurement
Pressure, in science (physics and chemistry) defined as the physical force or stress, applied perpendicular to the surface of objects per unit area. Mathematically, when F force is applied perpendicular to the object containing area A, pressure (P) = Force (F)/Area (A). Such a formula is used to derive unit dimensions of the pressure. For example, in the SI system unit of P = newton/metre2 (N/m2) or Pascal (Pa). The above formula is also used for the measurement of pressure in any state of matter such as gases, solids, and liquids.
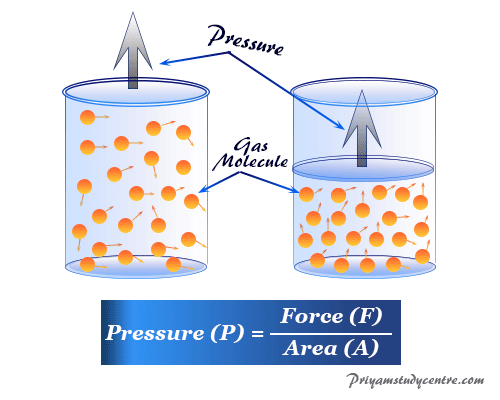
Measurement of Gas Pressure
A gas consists of a large number of very small spherical tiny particles, which are identified as molecules. If the thermal energy is much greater than the force of attraction, then we were given the matter in its gaseous state.
Molecules in the gaseous state move at very large speeds and the forces of attraction amongst them are not sufficient to bind them to one place. The fact indicated that the moves are practically independent of each other. Due to such features, gases are characterized by marked sensitivity of volume change with the change of T and P.
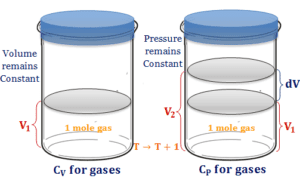
The molecules of a given gas are completely identical in size, shape, and mass with rapid or random motion. During their motion, they collide with each other and the wall of the container. Therefore, the pressure of the gas is developed due to the collisions of the molecules with the side of the vessel or container.
According to the ideal gas law, with decreasing volume, the gas molecule comes closer, or the collision between the gas molecules increases. Hence the T and P of the gas molecule increses.
Unit and Dimensions
The SI unit of force is Newton and the area is metre2, therefore the unit of pressure = Newton/metre2 (N/m2) or Pascal (Pa). The name of this unit was added in 1971 and before 1971, in the SI system, it was expressed simply in newtons per square metre.
Again the dimension of P is equal to the dimension of force/dimension of the area and it is equal to [M L−1 T−2].
At standard state, it is defined by the unit bar and 1 bar = 105 N m−2. It can also be expressed by different types of units,
- Millimetres of mercury
- Pounds per square inch (psi)
- In the CGS system dynes per square centimetre
- Millibars (mb)
- Standard atmospheres
- Kilopascals
Types of Pressure
It has four types such as atmospheric, absolute, differential, and gauge pressure in science.
- The air of the earth’s atmosphere is surrounded by different layers of gases that exert a force per unit area known as atmospheric pressure. It has a value close to 101325 Pascal (Pa). It decreases with increasing height from the surface of the earth. We use a mercury barometer to measure atmospheric pressure.
- Absolute pressure is the sum of atmospheric or barometric and gauge pressure. It is measured against the atmospheric or barometric pressure.