Electrical Resistance and Conductance
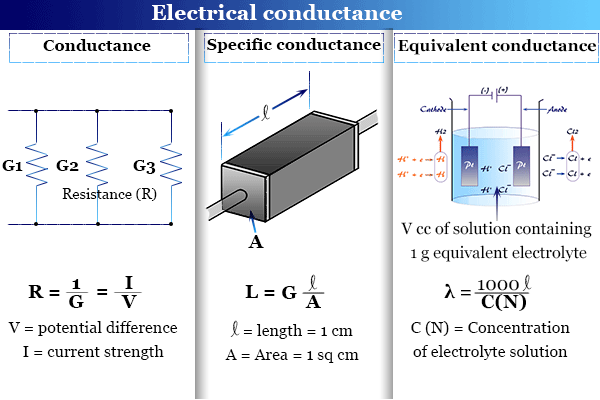
Conductance or electrical conductance is the reciprocal of resistance and describes the property of an electrolyte solution that helps to conduct electricity in the solution. Therefore, conductance is defined by a mathematical formula, G = 1/R = I/V (from Ohm’s law, R = V/I). Where, V = potential difference between the two electrodes dipped into the electrolytic solution, I = current strength, and R = electrical resistance that obstructs the flow of current. In chemistry or physics, the unit of conductance is equal to ohm−1 or mho and siemens in the SI system. The conducting ability of the electrolyte solution generally provides direct proof of the existence of ions in the solution. Therefore, the experimental determination of conductance provides the behavior of ions in the solution.
The electrical resistance and conductance of a given solution depends on,
- The nature of the electrolyte solution.
- The distance of separation of two electrodes.
- The area of electrodes.
Specific Conductance
The specific conductance (L) of an electrolytic solution is the conductance of the solution enclosed between two electrodes 1 cm apart and 1 cm2 area. In other words, the L may be defined as the conductance of 1 cc solution enclosed between the electrodes 1 cm apart. The unit of L is equal to ohm−1 cm−1 or Siemens m−1.
Specific conductance (L) depends on,
- The number of ions present per cc of the solution.
- The charge of the ions.
- The speed of the ions.
- It also depends on the temperature of the electrolytic solution and increases by a 2 percent per degree rise in temperature.
Therefore, with the dilution of the strong electrolyte solution, the number of ions per cc decreases while the speed of ions increases. Here the first factor predominates over the second and L decreases with the dilution of the solution.
Equivalent Conductance
It is defined as the conductance of a solution containing 1 gram equivalent electrolyte when the solution placed between two parallel electrodes 1 cm apart.
When the concentration of the electrolyte solution = C (N). The volume of the solution containing 1 gram equivalent electrolyte, V = 1000/C (N) cc.
The equivalent conductance
λ = V × L
or λ = 1000L/C (N)
Equivalent conductance unit
The unit of λ is equal to ohm−1 cm2/gram equivalent or Siemens m2/gram equivalent.
It is used to compare the conductance of different electrolyte solutions. Since the amount of electrolyte or 1 gram equivalent is fixed for λ.
Molar conductivity
Molar conductivity (μ) is defined as the conductivity of a solution containing 1 mole electrolyte when the solution is placed between two parallel electrodes 1 cm apart.
The unit of μ = ohm−1 cm2 mole−1 or Siemens m2 mole−1
Kohlrausch Law
The strong electrolyte solution is fully dissociated in any concentration. Therefore, the total number of ions fixed in 1 gram equivalent electrolyte solution is always fixed. With the dilution, the speed of the ions increases due to less interionic attraction among the ions.
At a certain dilution, the interionic attraction forces vanish, and λ riches limiting values. It is called equivalent conductance at infinite dilution (λ0). A strong electrolyte solution always obeys the Kohlrausch law of independent migration.
Kohlrausch Law of infinite dilution
Kohlrausch equation for strong electrolyte,
λc = λ0 − k√C
Where k = constant that depends on the temperature and dielectric constant (D) of the medium.
For electrolysis of weak electrolyte solution, the Kohlrausch formula or plot is not used for the determination of λ0. The law states that at infinite dilution, each ion moves independent of the influence of its co-ion and also contributes a definite share to the λ0 of the electrolyte.
Application of Conductivity Measurement
- The conducting ability of the electrolyte solution is the direct proof of the existence of ions in the solution. Therefore, the experimental determination of conductance provides the behavior of ions in the solution.
- The electrical conductance measurement is mostly used for the determination of various physical quantities like the endpoint in an acid base titration and the solubility of sparingly soluble salts. It is also used to measure the ionic product of water, and the chemical kinetics of the reactions.
- The electrical conductivity measurement of soil is extremely important for the health and growth of crops in the agricultural industry. Therefore, it is used to monitor essential nutrients of soil like phosphates, nitrates, calcium, and potassium which are used for plant growth.
- Conductance also offers a very simple and convenient way of determining the solubility of a sparingly soluble salt like silver chloride (AgCl).