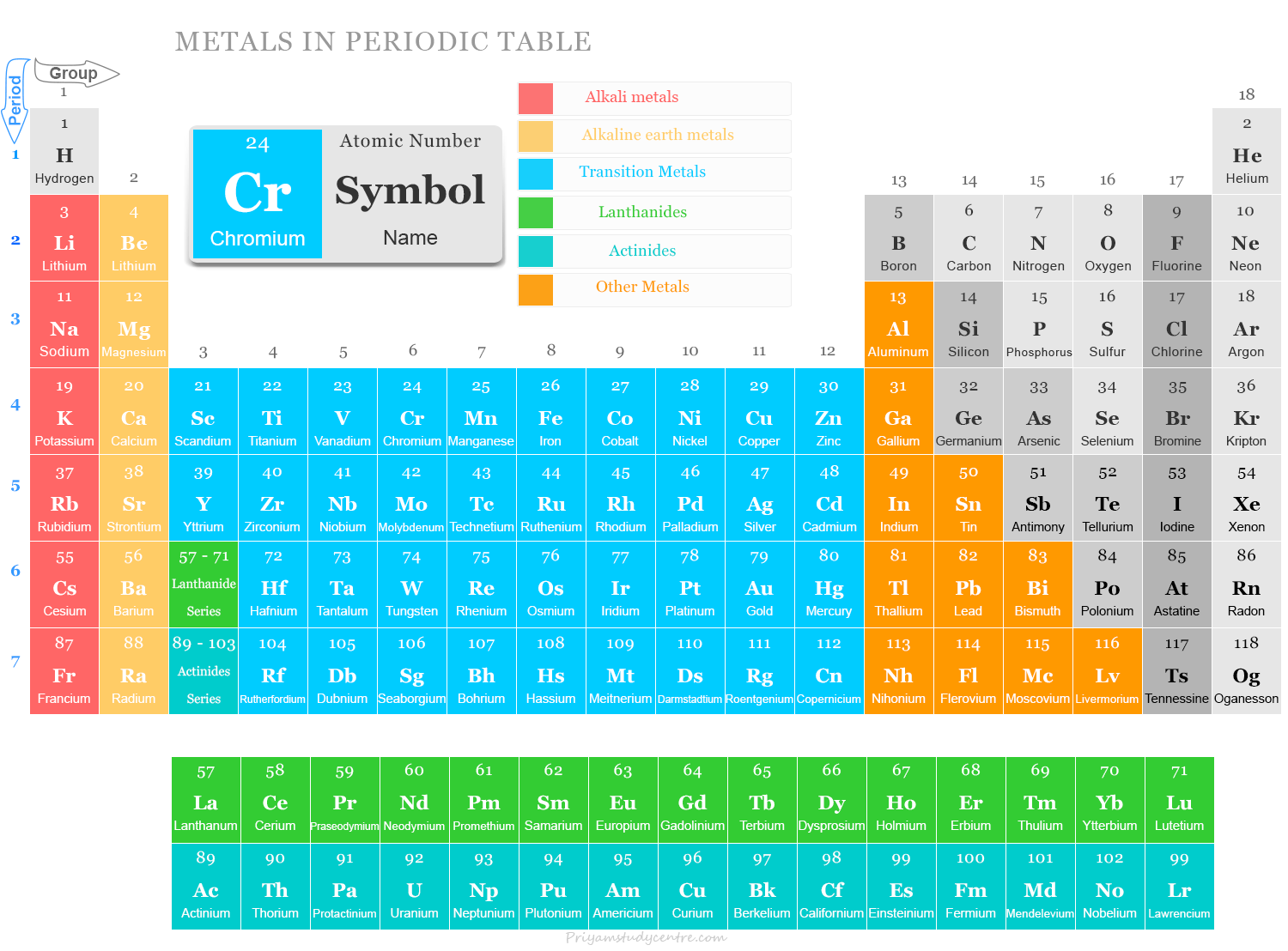
Metals in Periodic Table of Elements
Metals in chemistry are chemical elements that are good conductors of heat and electricity and form positive ions by losing electrons. On the basis of properties, 118 chemical elements present in the periodic table can be divided into two main types metal and non-metal. Metal is a substance that is produced naturally on our earth’s surface. Around 95 of the 118 elements in the periodic table are classified as metals.
Most of the metal is very strong and durable. Therefore, they are used for making various daily items such as automobiles, satellites, cooking utensils, etc. Iron, aluminum, copper, silver, and gold are familiar examples of metals that are used widely in our everyday lives.
Types of Metals in Periodic Table
93 out of 118 elements in the modern periodic table are metals and the remaining 18 are nonmetals and 7 are metalloids. Most nonmetals are gases and most metals are solid in standard temperature and pressure.
All the metals present in the periodic tables are classified into the following six types:
- Alkali metals
- Alkaline earth metals
- Transition metals
- Basic metals
- Lanthanides (rare earth elements)
- Actinides
Alkali Metals in Periodic Table
Alkali metals are the members of s-block elements and are present on the leftmost side of the periodic table. All these metallic elements are shiny, soft, highly reactive metals at standard temperature and pressure.
They readily lose their outermost electron to form unipositive cations with charge +1. Along with hydrogen, they constitute group 1 of the periodic table.
| Atomic Number | Name of the Metal | Symbol | Electronic configuration |
| 3 | Lithium | Li | [He] 2s1 |
| 11 | Sodium | Na | [Ne] 3s1 |
| 19 | Potassium | K | [Ar] 4s1 |
| 37 | Rubidium | Rb | [Kr] 5s1 |
| 55 | Caesium | Cs | 6s1 |
| 87 | Francium | Fr | [Rn] 7s1 |
Alkaline Earth Metals in Periodic Table
The alkaline earth metals are six chemical elements (beryllium, magnesium, calcium, strontium, barium, and radium) in group 2 of the periodic table. All elements of group 2 are shiny, silvery-white, and readily loose to form cations with a charge of +2 and an oxidation state of +2. Therefore, they are reactive metals at standard temperature and pressure.
The atomic number, name, symbol, and valence shell electronic configuration of alkaline earth metals are given below the table,
| Atomic Number | Name of the Metal | Symbol | Electron Configuration |
| 4 | Beryllium | Be | [He] 2s2 |
| 12 | Magnesium | Mg | [Ne] 3s2 |
| 20 | Calcium | Ca | [Ar] 4s2 |
| 38 | Strontium | Sr | [Kr] 5s2 |
| 56 | Barium | Ba | [Xe] 6s2 |
| 88 | Radium | Ra | [Rn] 7s2 |
Transition Metals in Periodic Table
In learning chemistry, a transition metal is an element that contains partly filling d orbitals in its atomic state or any of its common oxidation states.
A transition metal is a chemical element in the d-block of the periodic table. Therefore, the metals lying between s- and p-block elements of the periodic table are collectively called transition metals.
The atomic number, name, and symbol of d-block elements are given below the table,
| 3d-Block | ||||||||||
| 4d-Block | ||||||||||
| 5d-Block | ||||||||||
| 6d-Block |
Basic Metals
The basic metals are located to the right of the transitional metals on the periodic table. They are also called post-transitional metals. Such types of metals conduct heat and electricity. Like other metals, they form a metallic luster and tend to be dense, malleable, and ductile.
The atomic number, name, and symbol of basic or post-transition metals are listed below the table:
| Atomic number | Symbol | Name of the metal | Electronic configuration |
| 13 | Aluminum | Al | [Ne]3s23p1 |
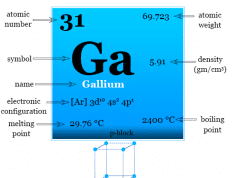
| 31 | Gallium | Ga | [Ar]3d104s24p1 |
| 49 | Indium | In | [Kr]4d105s25p1 |
| 50 | Tin | Sn | [Kr]4d105s25p2 |
| 81 | Thallium | Tl | [Xe]4f145d106s26p1 |
| 82 | Lead | Pb | [Xe]6s24f145d106p2 |
| 83 | Bismuth | Bi | [Xe]6s24f145d106p3 |
| 113 | Nihonium | Nh | [Rn]5f146d107s27p1 (predicted) |
| 114 | Flerovium | Fl | [Rn]5f146d107s27p2 (predicted) |
| 115 | Moscovium | Mc | [Rn]5f146d107s27p3 (predicted) |
| 116 | Livermorium | Lv | [Rn]5f146d107s27p4 (predicted) |
| 117 | Tennessine | Ts | [Rn]5f146d107s27p5 (predicted) |
f-Block Elements
The metals in which the additional electron enters (n−2)f orbitals are called inner transition metals. They are also called f-block elements because the extra electrons go to the f-orbitals belonging to (n−2) the main shell.
f-block elements are classified into two categories such as 4f-block and 5f-block elements.
Rare Earth Metals on the Periodic Table
4f-block elements are also called lanthanides, lanthanones, or rare-earth metals. The first two names are given because their properties resemble lanthanum.
The name rare earth was given because they were extracted from oxides present in the earth and were considered to be rare. Presently, the name rare earth is avoided because many of these elements are no longer rare but are abundant.
Today, ion exchange and solvent extraction processes are used for quick production of highly pure, low-cost rare earths. However, the old name is still in use to describe such metals.
| Atomic Number | Name | Symbol | Electronic configuration |
| 57 | Lanthanum | La | [Xe]4f05d16s2 |
| 58 | Cerium | Ce | [Xe]4f15d16s2 |
| 59 | Praseodymium | Pr | [Xe]4f36s2 |
| 60 | Neodymium | Nd | [Xe]4f46s2 |
| 61 | Promethium | Pm | [Xe]4f56s2 |
| 62 | Samarium | Sm | [Xe]4f66s2 |
| 63 | Europium | Eu | [Xe]4f76s2 |
| 64 | Gadolinium | Gd | [Xe]4f75d16s2 |
| 65 | Terbium | Tb | [Xe]4f96s2 |
| 66 | Dysprosium | Dy | [Xe]4f106s2 |
| 67 | Holmium | Ho | [Xe]4f116s2 |
| 68 | Erbium | Er | [Xe]4f126s2 |
| 69 | Thulium | Th | [Xe]4f136s2 |
| 70 | Ytterbium | Yb | [Xe]4f146s2 |
| 71 | Lutetium | Lu | [Xe]4f145d16s2 |
5f-Block Elements
The elements in which the extra electron enters 5f-orbitals of (n−2) main shell are called 5f-block elements, actinides, or actinones.
The atomic number, name, symbol, and electronic configuration of 5f-block elements are given below,
| Atomic number | Name | Symbol | Electronic Configuration |
| 90 | Thorium | Th | [Rn]6d27s2 |
| 91 | Protactinium | Pa | [Rn]5f26d17s2 |
| 92 | Uranium | U | [Rn]5f36d17s2 |
| 93 | Neptunium | Np | [Rn]5f46d17s2 |
| 94 | Plutonium | Pu | [Rn]5f67s2 |
| 95 | Americium | Am | [Rn]5f77s2 |
| 96 | Curium | Cm | [Rn]5f76d17s2 |
| 97 | Berkelium | Bk | [Rn]5f97s2 |
| 98 | Californium | Cf | [Rn]5f107s2 |
| 99 | Einsteinium | Es | [Rn]5f117s2 |
| 100 | Fermium | Fm | [Rn]5f127s2 |
| 101 | Mendelevium | Md | [Rn]5f137s2 |
| 102 | Nobelium | No | [Rn]5f147s2 |
| 103 | Lawrencium | Lr | [Rn]5f147s27p1 |
Properties of Metals
Based on properties, elements present in the periodic table can be divided into two main groups metals and non-metals. The atoms of metallic substances are arranged commonly in one of three common crystal structures such as body-centered cubic (bcc), face-centered cubic (fcc), and hexagonal close-packed (hcp).
For example, atoms of chromium, iron, and tungsten are arranged by body-centered cubic crystal structures while atoms of aluminum, copper, and gold are arranged by body-centered cubic crystal structures. The hexagonal close-packed crystalline solid is found in titanium, cobalt, and zinc.
Metallic Bonding
Metallic bonding is a type of chemical bonding that helps to hold metallic atoms together by an electrostatic force of attraction between conduction or delocalized electrons and positively charged metal ions.
The outer electrons in a metal atom can delocalized over the whole metal structure. They are not attached to a particular atom or pair of atoms but they move freely around the whole structure. Therefore, metallic bonding is often called an array of positive ions in a sea of electrons.
The strength of metallic bonds is different for different metallic elements. It reaches a maximum around the middle of the transition metals series because they contain large numbers of delocalized electrons.
Metallic Lustre
In their pure state, most of the metals have bright shining surfaces. This metallic property is called metallic lustre. In other words, metallic luster is a property that describes the amount of light that reflects off the metal.
Metallic elements like gold, silver, and platinum are known for their shining surface. Gold is shining yellow, silver is shining white, and copper is brown, iron, aluminum and zinc are lustrous grey.
Hardness of Metal
Most commonly, a metal is hard in nature and the hardness varies from metal to metal. All the metals except mercury exist in solid at room temperature. Some alkali metals like lithium, sodium, and potassium are very soft and they can be easily cut by a knife.
Ductility of Metals
A metal is generally ductile in nature. It is the property due to which a metal can be drawn into thin wire. Gold is the most ductile metal found in our earth.
Malleability Metals
Generally, a metal is malleable in nature. It is the property due to which a metal can be beaten into thin sheets. Gold and silver are the most malleable metallic elements in the periodic table.
Electrical Conductivity
Generally, a metal is a good conductor of electricity in the solid state. However, the conductivity varies from one metal to another. According to the band theory, a conductor has to have its partially filled uppermost energy band. Therefore, under an applied filled gradient electrons can move from a partially filled band to vacant sites in the same energy band.
The electrical conductivity of a metal depends not on the number of electrons it has but on the number for which vacant levels in an energy band are available. The conduction of electricity or flow of electric current in metal occurs due to the flow of free electrons present in the metal. Semiconductors and nonmetals are relatively poor conductors because they have a very small energy gap between the filled band and the vacant band.
The transition elements copper, silver, and gold have a d10 s1 electronic configuration. In the metallic state, they all have a filled d energy band but only a half-filled s band. Therefore, vacant sites in the s band are available for electronic movement.
Other transition elements have partially filled d orbitals. Therefore, in the metallic state, they have partially filled d energy bands. It explains the higher electrical conductivity in transition metals.
Good Conductor of Heat
The delocalized electrons in metals are highly mobile. Therefore, they are easily able to pass on heat-induced vibrational energy.
Except for lead and mercury, metals are good conductors of heat. Lead and mercury are poor conductors of heat but copper and silver are the best conductors of heat.
Melting and Boiling Points
The atoms in metals have a strong attractive force between them and much energy is required to overcome it. Therefore, a metal generally has very high melting and boiling points.
Tungsten has the highest melting point among all metals in the periodic table while gallium and cesium have very low melting points. Therefore, gallium and caesium will melt when we keep them in our plums.
Sonority of Metals
The metals that produce a sound on striking hard surfaces are called sonorous. Using this property, a metal can be used for making school bells.
Chemical Reactions of Metals
Metals are commonly formed cations by losing their valence electrons. Most of the metals present in the periodic table react with oxygen, water, and acids.
The chemical reactions of metals depend on the reactivity of the metal. For example, alkali and alkaline earth metals show high reactivity and react vigorously with dilute acids, oxygen, and water.
Reaction of Metals with Oxygen
Almost all metals combine with oxygen or air to form metal oxides.
Metal + Oxygen → Metal oxide
Different metals react with oxygen at different rates. For example, sodium (Na) and potassium (K) react vigorously while magnesium (Mg) and aluminum (Al) burn in air only by heating.
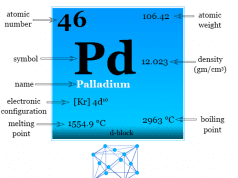
Zinc (Zn) burns only on strong heating while iron (Fe) does not burn in the form of a rod or block. The metal copper does not burn on heating but blister copper burns. The noble metals (palladium, platinum, and gold) do not react with oxygen even at high temperatures.
Aluminum forms aluminum oxide when heated in air.
4 Al (s) + 3 O2 (g) → 2 Al2O3 (s)
Similarly, when copper is heated in air, it combines with oxygen to form black copper (II) oxide.
2 Cu + O2 → 2 CuO
Properties of Metal Oxides
Generally, metal oxides are basic in nature but some metal oxides such as aluminium oxide and zinc oxide show both acidic and basic properties.
Such metal oxides which react with both acids and bases to form salt and water are called amphoteric oxides. Therefore, aluminum oxide and zinc oxide are examples of amphoteric oxides.
Metallic oxides are insoluble in water but alkali metal oxides are dissove in water to form corresponding hydroxides. For example, sodium hydroxide and potassium hydroxide dissove in water to form sodium hydroxide and potassium hydroxide respectively.
Na2O (s) + H2O (l) → 2 NaOH (l)
K2O (s) + H2O (l) → 2 KOH (l)
Reactions of Metal with Water
Generally, a metal reacts with water to form a metal oxide and hydrogen gas. The metal oxides which are soluble in water further react with water to form metal hydroxide.
Metal + Water → Metal oxide + Hydrogen gas
Metal oxide + Water → Metal hydroxide
Reaction with Highly Reactive Metals
The metallic elements potassium and sodium react violently with cold water. In the case of sodium and potassium, the reaction is extremely violent and exothermic.
2 K + 2 H2O → 2 KOH + H2 + Heat
2 Na + 2 H2O → 2 NaOH + H2 + Heat
The heat evolved is sufficient for hydrogen to catch fire. Therefore, Na and K are kept in kerosene instead of water.
The reaction of alkaline earth metal calcium with water is less violent. The heat that evolved in this reaction is not sufficient for hydrogen to catch fire.
Ca + 2 H2O → Ca(OH)2 + H2
Calcium floats over water because the bubbles of hydrogen gas stick to the surface of the metal.
Reaction with Moderately Reactive Metals
Metallic elements such as aluminum, iron, and zinc do not react either with cold or hot water. They react with the stream and form a metal oxide and hydrogen gas.
2 Al + 3 H2O → Al2O3 + 3 H2
3 Fe + 4 H2O → Fe3O4 + 4 H2
Reaction with Non-reactive Metals
Lead, copper, silver, and gold do not react with water at all. Therefore, the reactivity order of metallic elements toward water is:
K > Na > Ca > Mg > Al > Fe > Pb > Cu > Ag > Au
Reactions with Acids
Except few less reactive metals, all react with dilute sulfuric acid and hydrochloric acid to produce salt and hydrogen gas.
Metal + Dilute acid → Salt + Hydrogen
Therefore, zinc reacts with dilute hydrochloric acid (HCl) to form zinc chloride (ZnCl2) and hydrogen gas
Zn + 2 HCl → ZnCl2 + H2
Due to the strong oxidizing nature of nitric acid, hydrogen gas does not evolve during the reaction of a metal with HNO3. Only magnesium and manganese react with very dilute nitric acid to evolve hydrogen gas.
Metal (Mg/Mn) → Salt + H2
Displacement Reaction of Metals
Reactive metals can displace a comparatively less reactive metal from its compounds in aqueous solution or molten form. The general chemical equation is:
Metal A + Salt of B → Salt of A + Metal B
Cu + 2 AgNO3 → Cu (NO3)2 + 2 Ag
The above type of metallic reaction is called the displacement reaction.
Uses of Metals
Metals are present in nearly all forms of modern life due to their strength, durability, and availability. Iron may be the most common heavy metal used widely in our daily life. Aluminum is the next most commonly light metal used also in many parts of life.
One or more of the essential and trace metals (iron, cobalt, nickel, copper, and zinc) are essential for all higher forms of life. For example, iron is an essential component of hemoglobin and cobalt is an essential component of vitamin B12 (Cobalamin).
The common and most popular uses of metals are included below:
- Construction industry
- Electronics
- Biology and biochemistry
- Machinery, refractory, and automobiles
- Decorative products and jewellery
- Other uses of metals
Frequently Asked Questions
What are metals in chemistry?
Metals in chemistry are substances that have high electrical conductance, luster, and malleability, which readily lose their valence electrons to form positive ions (cations).
Which element is the most metallic?
The most metallic element found in the periodic table is francium. However, francium is a man-made chemical element. However, francium is a man-made element and most isotopes of francium are radioactive they almost instantly decay to form another element. Therefore, the natural element which shows the highest metallic character is caesium.
How many elements on the periodic table are metals?
93 out of 118 elements in the modern periodic table are metals and the remaining 18 are nonmetals and 7 are metalloids. All the metallic elements present in the periodic tables are classified into the following six types:
- Alkali metals
- Alkaline earth metals
- Transition metals
- Basic metals
- Lanthanides (rare earth elements)
- Actinides