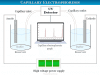
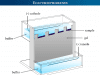
Water Electrolysis Equipment
Water electrolysis is the process where water can split into hydrogen (H2) and oxygen (O2) through the requirement of electricity or electrical energy in an electrolyzer equipment. Most of the hydrogen gas produced or used worldwide is created through the electrolysis of water in an electrolyzer. The Dutch merchants Jan Rudolph Deiman and Adriaan Paets van Troostwijk first represented the electrolysis of water in 1789. They use an electrostatic machine to make electricity and discharge between two gold electrodes immersed in water.
After the development of Volta’s battery technology by Johann Wilhelm Ritter separate product gases (hydrogen and oxygen).
Almost a century later, in 1888, a process of industrial production of hydrogen and oxygen via electrolysis of water was developed by the Russian engineer Dmitry Lachinov.
In 1902 more than 400 industrial water electrolyzers were used to produce hydrogen and oxygen via electrolysis. These electrolyzers utilized aqueous alkaline solutions (sodium hydroxide or potassium hydroxide solution) as their electrolytes. Such electrolysis techniques carry on to the present day for the production of hydrogen and oxygen gases from water.
Water Electrolysis Energy Requirement
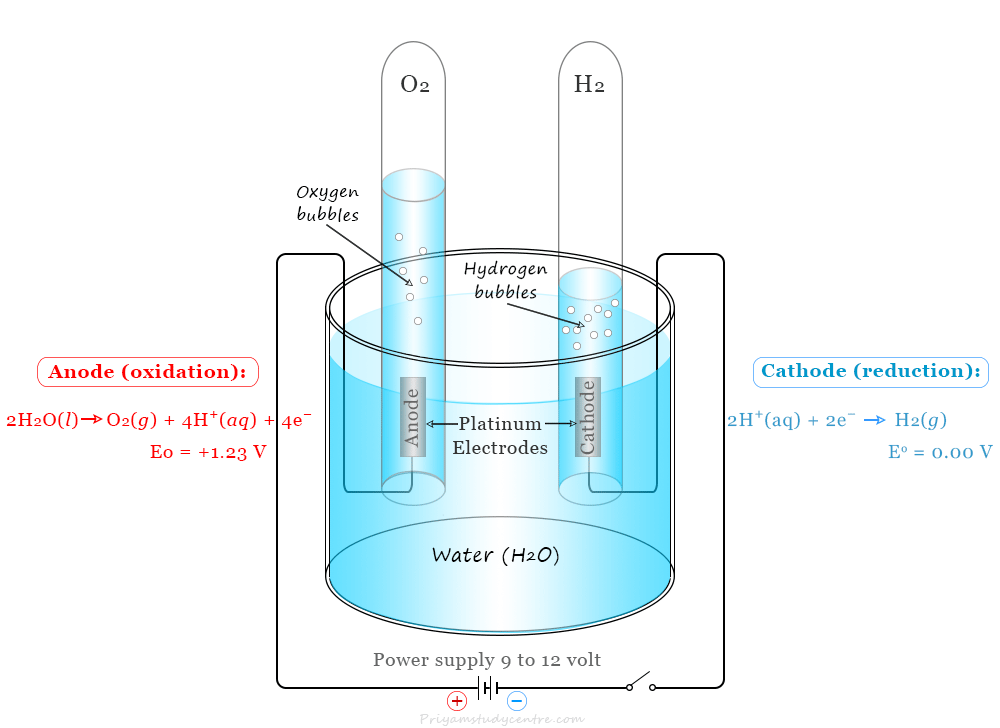
The basic principle of water electrolysis equipment is carried out by splitting water into oxygen and hydrogen with the help of a DC electrical power source. Two electrodes or two plates which are commonly made from an inert metal such as platinum or iridium are placed in the water.
The splitting occurs in two half-cell reactions that take place at the two electrodes in the electrochemical cell. Therefore, when a DC electrical power source is connected to two electrodes, hydrogen appears at the cathode, and oxygen appears at the anode.
Water Electrolysis Reaction
Electrolysis is a technique that uses direct current (DC) for chemical reactions in two electrodes (cathode and anode) dipped into the electrolyte solution.
The electrolytic cell contains a pair of platinum electrodes immersed in water to which a small amount of an electrolyte such as H2SO4, NaOH, or KOH has been added. The electrolyte is necessary because pure water is a very poor conductor of electricity and dissociates H+ and OH− to a negligible extent.
- In the negatively charged cathode, a reduction reaction takes place, and electrons (e−) from the cathode are given by hydrogen cations (H+) to form hydrogen gas.
- In the positively charged anode, an oxidation reaction occurs by giving electrons to the anode, generating oxygen gas. Therefore, two half-reactions take place in two electrodes.
Pure Water Electrolysis
Pure water molecules are very poor conductors of electricity and dissociate H+ and OH− to a negligible extent. Therefore, no measurable current flow occurs in equipment when the electrodes are connected to the power supply during water electrolysis.
An excess of energy in the form of overpotential is usually required for the electrolysis of pure water. Such overpotential requires overcoming activation energy, ion mobility (diffusion) and concentration, entropy, etc.
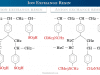
Pure water molecules (pH = 7) have limited self-ionization and decomposition at standard temperature and pressure is not favorable in thermodynamic terms. At 25°, the half-reactions of pure water (pH = 7) during electrolysis:
Anode (oxidation):
2 H2O (l) → O2 (g) + 4 H+ + 4 e– E° = +1.23 V
Cathode (reduction):
4 H+ + 4 e– → 2 H2 (g) + 4 e– E° = 0.00 V
Overall Reaction:
2 H2O (l) → 2 H2 (g) + O2 (g) E° = −1.23 V
The standard potential of the pure water electrolysis is negative and hence is the thermodynamically unfavorable process. Therefore, due to the low concentration of ions and the interface crossed electrons, an extra voltage (overvoltage) is needed during electrolysis. However, the addition of a little electrolyte (acid/base/salt) increses the conductivity of water.
Acidified Water Electrolysis
In an acidic medium, water molecules can dissociate into hydrogen (H+) and hydroxyl (OH−) ions. In the presence of acid, the half-reactions of electrolysis of water are:
Cathode (reduction):
4 H+ (aq) + 4 e− → 2 H2 (g)
Anode (oxidation):
2 H2O (l) → O2 (g) + 4 H+ (aq) + 4 e−
Overall Reaction:
2 H2O (l) → 2 H2 (g) + O2 (g)
Alkaline Water Electrolysis
Similarly, in an alkaline medium, water molecules can dissociate into hydrogen (H+) and hydroxyl (OH−) ions. In the presence of a base, the two half-reactions in water electrolysis are:
Cathode (reduction):
4 H2O (l) + 4 e− → 2 H2 (g) + 4 OH− (aq)
Anode (oxidation):
4 OH− (aq) → O2 (g) + 2 H2O (l) + 4 e−
Overall Reaction:
2 H2O (l) → 2 H2 (g) + O2 (g)
Electrolytes for Water
Pure water is a very poor conductor of electricity and dissociates H+ and OH− to a negligible extent. Therefore, the charge carrier density of pure water is very low and similar to that of semiconductors.
The conductivity of water increses considerably in the presence of an electrolyte because it disassociates into cations and anions in water. The electrolyte can also enhance the dissociation of water to H+ and OH−. Therefore, a small amount of an acid/base/salt electrolyte has been added during the electrolysis of water.
Acidic Electrolytes
When a small quantity of mineral acid is added, the H+ ion obtained from acid has no competitor for the H+ is created by disassociating water. Therefore, electrons (e−) from the cathode are given by H+ ions to form hydrogen gas.
The hydroxide ions from water can oxidize when acid anions have high standard electrode potential. However, when the negative part of an acid (electrolyte) has less standard electrode potential than the hydroxide ion, the negative part of the acid will be oxidized instead of the hydroxide ion. Therefore, there is no producing any oxygen gas.
Sulfuric acid is a good choice during the electrolysis of water because the standard potential of sulfate ions is much higher than hydroxide ions. Therefore, oxygen gas is produced in the anode during electrolysis of water.
Basic Electrolytes
When a small quantity of a base (electrolyte) is added, a cation is formed along with the hydrogen ion during the dissociation of the aqueous electrolyte. Therefore, a hydrogen ion can be reduced when a cation obtained from electrolytes has a lower standard electrode potential than a hydrogen ion.
Various alkali metal cations (Li+, Na+, Rb+, K+, Cs+) and alkaline earth metal cations (Ba2+, Sr2+, Ca2+, Mg2+) have lower electrode potential than H+. Therefore, they are suitable for use as electrolyte cations. Sodium and potassium are the most common choices during the electrolysis of water because they are inexpensive and form water-soluble salts.
Salt Electrolytes
Various water-soluble salts are used as an electrolyte during electrolysis of water. The most common salt electrolytes may include sodium chloride (NaCl), potassium chloride (KCl), sodium or potassium bicarbonate, etc. The electrolysis of these salt solutions gives hydrogen gas at the cathode along with other gases instead of oxygen gas in the anode.
- For example, electrolysis of aqueous sodium chloride or potassium chloride solution produces hydrogen gas at the cathode and chlorine gas at the anode.
- Similarly, electrolysis of aqueous sodium or potassium bicarbonate solution produces hydrogen gas at the cathode and carbon dioxide gas at the anode.
Electrolyzer for Water
An electrolyzer is an electrolytic equipment where hydrogen and oxygen are separated from water by electrolysis.
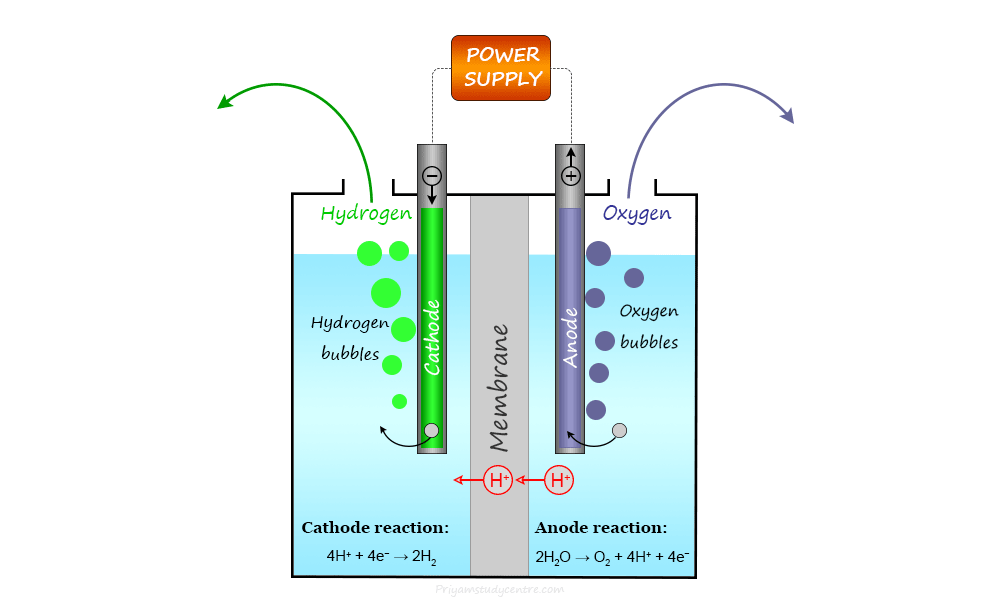
Depending on the transporter of the electrolyte, electrolyzers can be divided into three types−polymer electrolyte membrane (PEM) electrolyzer, alkaline electrolyzer, and solid oxide electrolyzer.
Polymer Electrolyte Membrane Electrolyzers
The electrolyte is in a solid state in a polymer electrolyte membrane (PEM) electrolyzer.
At the anode, water can react to form oxygen gas and positively charged hydrogen ions (protons).
Anode reaction:
2 H2O → O2 + 4 H+ + 4 e−
The electrons flow through an external circuit and the hydrogen ions selectively move across the polymer electrolyte membrane (PEM) to the cathode where hydrogen ions combine with electrons to form hydrogen gas.
Cathode reaction:
4 H+ + 4 e− → 2 H2
Alkaline Electrolyzer
An alkaline water electrolyzer is a type of electrolyzer that operates by the transport of hydroxide ions (OH−) through the electrolyte from the cathode to the anode. Generally, a liquid alkaline solution of sodium hydroxide (NaOH) or potassium hydroxide (KOH) is used as an electrolyte in an alkaline water electrolyzer.
Alkaline electrolyzers have been commercially available for many years. The alkaline electrolyzers commonly contain a diaphragm that transports the hydroxide ions (OH−) from one electrode to the other and separates product gases. A recent comparison study shows that solid alkaline exchange membrane-based water electrolyzers had similar or better efficiencies than polymer electrolyte membrane (PEM) based electrolyzers.
Solid Oxide Electrolyzer
The electrolyte in a solid oxide electrolyzer is a ceramic material that selectively conducts negatively charged oxygen ions at high temperatures (about 700°–800°C). At the cathode, steam is reduced to form hydrogen and oxide ions which pass through the solid ceramic membrane. It oxidized at the anode to form oxygen gas and electrons for the external circuit.
Solid oxide electrolyzers can be used at high temperatures to reduce the external voltage needed for electrolysis of water. Advanced lab-scale solid oxide electrolyzers based on proton-conducting ceramic electrolytes operate at lower temperatures of 500°–600°C.
Applications of Electrolysis of Water
The production of pure hydrogen and oxygen is the main application of the electrolysis of water. Generally, water electrolysis equipment can be used to generate oxygen for the International Space Station (ISS), production of hydrogen for the chemical industry and renewable energy generation, production of deuterium from heavy water, etc.
Hydrogen From Water Electrolysis
A large quantity of hydrogen gas generated worldwide is created by water electrolysis equipment. Most of the hydrogen gas produced through electrolysis is a by-product of the production of chlorine and caustic soda.
For example, in the chloralkali process, the electrolysis of aqueous sodium chloride (a brine) in a membrane cell produced chlorine, caustic soda (NaOH), and hydrogen gas.

A membrane (Nafion, Flemion, or Aciplex) is used to prevent the mixing of chlorine and hydroxide ions.
2 NaCl + 2 H2O → Cl2 + H2 + 2 NaOH
- In the first chamber, the chloride ions from the brine solution are oxidized at the anode by losing electrons to form chlorine gas. Therefore, sodium ions from sodium chloride are released in the solution.
2 Cl− → Cl2 + 2 e− - In the second chamber, positive hydrogen ions pulled from water molecules are reduced by gaining electrons to form hydrogen gas. Therefore, hydroxide ions present in water molecules are released in the solution.
2 H2O + 2 e− → H2 + 2 OH− - The ion-exchange membrane present in the center of the cell allows the sodium ions (Na+) to pass to the second chamber. Therefore, sodium ions from brine react with hydroxide ions from water to produce caustic soda (NaOH).
Na+ + OH− → NaOH
Oxygen from Water Electrolysis
Water electrolysis equipment can be used to generate oxygen for the International Space Station (ISS). In a space station, oxygen and water systems contain two main components water reclamation system (WRS) and the oxygen generation system (OGS)
- Water reclamation system (WRS): The WRS system collects water and purifies it to potable standards from urine, humidity, and condensation. It makes up only a portion of the water aboard the International Space Station. Some water is also continually shipped from Earth to the station for the production of oxygen.
- Oxygen generation system (OGS): The OGS system designed by NASA and the Russian Elektron system split water into its elemental components (hydrogen and oxygen) by the process of electrolysis. Most of the electrical energy required for water electrolysis in the International Space Station comes from solar energy equipment through solar panels on the station’s exterior.
Production of Heavy Water
A small quantity of deuterium is naturally present in normal water. Therefore, the electrolysis of water produces oxygen and hydrogen along with deuterium. The hydrogen can be liquified and distilled to separate deuterium. The deuterium produced by this process reacted with oxygen to form heavy water (D2O).
Production of pure heavy water requires large electrolysis chambers and consumes large amounts of power. Therefore, the Girdler sulfide process is generally preferred.
Frequently Asked Questions (FAQs)
What is the electrolysis of water?
Electrolysis of water is the process where water can split into hydrogen (H2) gas and oxygen (O2) gas with the help of a DC electrical power source. Therefore, a large quantity of the hydrogen gas produced or used worldwide is created through the process of water electrolysis.
The electrolytic cell contains a pair of platinum electrodes dipped in water to which a small amount of an electrolyte such as H2SO4, KOH, or NaOH has been added.
What is the chemical equation for the electrolysis of water?
Electrolysis of water is a decomposition reaction that occurs in an acidic or basic medium. In an acidic medium, the two half-cell equations at the cathode and anode during water electrolysis are:
Cathode (reduction):
4 H+ (l) + 4 e− → 2 H2 (g)
Anode (oxidation):
2 H2O (l) → O2 (g) + 4 H+ (aq) + 4 e−
In an alkaline medium, the two half-cell equations at the cathode and anode during water electrolysis are:
Cathode (reduction):
4 H2O (l) + 4 e− → 2 H2 (g) + 4 OH− (aq)
Anode (oxidation):
4 OH− (aq) → O2 (g) + 2 H2O (l) + 4 e−
Overall equation:
2 H2O (l) → 2 H2 (g) + O2 (g)
Which water has electrolytes?
Pure water is a very poor conductor of electricity and dissociates H+ and OH− to a negligible extent. The conductivity of water increses considerably in the presence of an H2SO4 because it disassociates into cations and anions in water.
Sulfuric acid can also enhance the dissociation of water to H+ and OH−. Therefore, a small amount of sulfuric acid has been added during the electrolysis of water.
Can pure water be electrolyzed?
Pure water molecules (pH = 7) are very poor conductors of electricity and dissociate H+ and OH− to a negligible extent. Therefore, an excess of energy in the form of overpotential is usually required for the electrolysis of pure water.
Without the excess energy, electrolysis occurs slowly or does not occur at all. Pure water molecules (pH = 7) have limited self-ionization. Therefore, decomposition at standard temperature and pressure is not favorable in thermodynamic terms.
What is water electrolyzer?
An electrolyzer is an electrochemical cell consisting of an anode and a cathode separated by an electrolyte membrane. Different electrolyzers function in different ways because of the different types of electrolyte material involved and the ionic species they conduct during electrolysis.
A water electrolyzer is also an electrochemical cell used for splitting water into hydrogen (H2) gas and oxygen (O2) gas with the help of a DC electrical power source. Polymer electrolyte membrane (PEM) electrolyzers, alkaline electrolyzers, and solid oxide electrolyzers are common types of electrolyzers used in the electrolysis of water.