Solution Definition in Chemistry
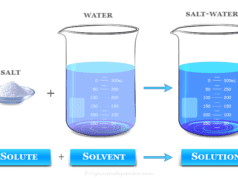
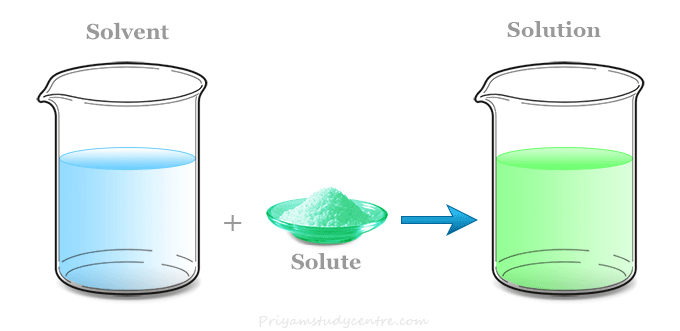
A solution in chemistry is a type of homogeneous mixture of two or more chemical substances where a minor component (solute) is uniformly distributed within the major component (solvent). When the solvent is water, we used the term aqueous solution. Chemical solutions may exist in any phase like solid, liquid, or gases. For example, a mixture of nitrogen and oxygen gases, an aqueous solution of sodium chloride or carbon dioxide, and a mixture of sodium and mercury in sodium amalgam. Solutions have two parts solute and solvent. The ability of a solute to dissolve in a solvent is called solubility. The solubility of a substance in a solvent depends on the nature of the solute and solvent. In general, polar solutes dissolve in polar solvents and nonpolar solutes dissolve in nonpolar solvents.
Solute in Chemistry
In chemistry, a solute is a substance or minor component that is dissolved in the solution to form a homogeneous mixture. Concentration is the measurement of solute present in a chemical solution with respect to solvent.
Examples of Solute
Commonly, a solute is solid but it may also exist as a liquid or gaseous form.
- In salt or sugar in water, solutes are solids which dissolve in liquid to form chemical solutions.
- In an aqueous solution of hydrogen chloride, carbon dioxide or ammonia, the solutes like hydrogen chloride or carbon dioxide or ammonia are in a gaseous state.
- When two liquids are mixed to form a solution, the solute can be identified by their concentration. The species which present in a smaller ratio is called the solute. For example, in 1 M hydrochloric acid, hydrochloric acid is the solute while water is the solvent.
- The terms solvent and solute are also applied for amalgams and metal alloys. For example, carbon is a solute in steel and mercury is a solute in zinc amalgam (Zn/Hg).
What is a Solvent?
A solvent is a substance (major component) of a solution in which a solute dissolves to form a homogeneous mixture. Commonly, a solvent is a liquid but it may also be a solid, gas, or supercritical fluid. The solvent may determine the physical state of the solutions. Water is the most common solvent for many chemical compounds.
Examples of Solvent
- Water, ethanol, methanol, and acetone are common examples of liquid solvents with the ability to dissolve many of the chemical compounds to form a solution.
- Air is an example of a gas-gas solution, where other gases are dissolved in nitrogen gas. Here nitrogen is a solvent because the amount of nitrogen present in the air is higher.
- Palladium or platinum is an example of a solid solvent because hydrogen gas is absorbed by these metals.
Types of Solution
Any state of matter (solid, liquid, or gas) can participate as a solute and as a solvent during the formation of a chemical solution. Therefore, depending on the physical states of the solute and solvent, the chemical solution may be classified into the following different types.
| Types of solution | Solute | Solvent | Examples |
| Gas-gas | gas | gas | A mixture of gases that do not undergo chemical reactions. For example, a mixture of air, hydrogen, and helium. |
| Gas-liquid | gas | liquid | An aqueous hydrogen chloride, aqueous carbon dioxide, aqueous ammonia, etc. |
| Gas-solid | gas | solid | Hydrogen is absorbed by platinum or palladium to form a gas-solid solution. |
| Liquid-gas | liquid | gas | Aerosol or water vapour in the air. |
| Liquid-liquid | liquid | liquid | A mixture of water and ethanol or a mixture of benzene and toluene. |
| Liquid-solid | liquid | solid | Combination of sodium and mercury in sodium-amalgam (Na/Hg) or a combination of sodium and zinc in zinc-amalgam (Zn/Hg) |
| Solid-liquid | solid | liquid | The aqueous sodium chloride, sugar, etc. |
| Solid-solid | solid | solid | Alloys like brass (a mixture of copper and zinc) or bronze (a mixture of copper and tin). |
| Solid | solid | gas | Camphor in nitrogen gas |
Characteristics of Solutions
The chemical solutions have the following characteristics,
- It is a homogenous mixture of solute and solvent.
- Generally, the radius of the solute molecule present in the solutions is 10-7 to 10-8 cm. Therefore, the solute cannot be visible to the naked eye.
- Different types of components present in a chemical solution cannot be separated by filtration.
- Generally, it is a single-phase homogeneous mixture.
- A light beam passes cleanly through a true chemical solution.
- In many cases, heat is absorbed or evolved during the formation of solutions. For example, the heat evolved when Ca(OH)2 is dissolved in water but heat is absorbed when NH4Cl is dissolved in water.
Concentration of a Solution
Concentration is an important term for chemical solutions. It can calculate how much solute is present in a chemical solution or solvent.
We used different types of units to derive the concentration of a solution. The common units which we used to derive the concentration are normality, molality, morality, mass percent, volume percent, and mole fraction.
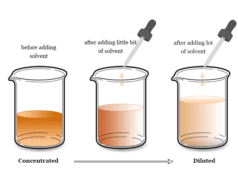
In chemistry, we always discuss concentrated and dilute solutions.
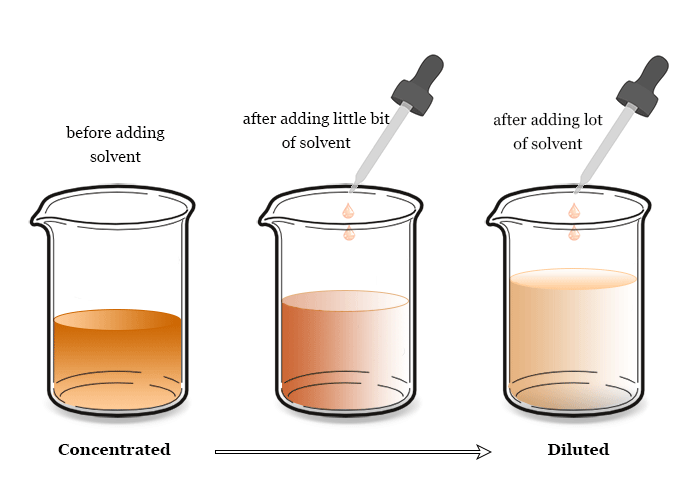
- A dilute solution is one where a small amount of solute is dissolved in a relatively large quantity of solvent.
- Similarly, a concentrated solution is one where a large amount of solute is dissolved in a solvent to form a chemical solution.