What is Electrode in Chemistry?
Electrode is the type of electronic conductor, usually metals partly immersed in an electrolytic solution, and imparts or receives electrons from the medium in a storage battery, solid, gas, or vacuum. Electrodes are used commonly in electrochemical cells, semiconductors like diodes, and different types of medical devices. In the electrolysis of electrochemical cells, the electrical energy from the external sources is used to perform a chemical change or redox reaction.
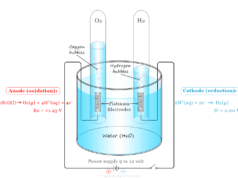
The negative electrode where oxidation occurs is called the anode and the positive electrode where reduction occurs is called the cathode in chemistry or chemical science.
Types of Electrodes
Current-carrying ions are discharged at the electrode of the chemical solution. In water solution, when negative ions are difficult to discharge due to high deposition potential and hydroxyl ion gets deposited.
When positive ions are difficult to be discharged, hydrogen ions are discharged from the solution. According to the reactivity, the electrodes are two types
- Inert electrodes
- Reacting electrodes
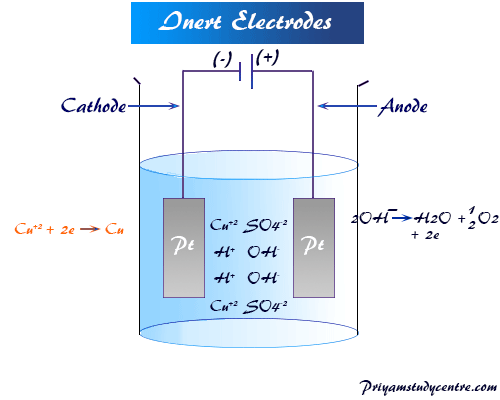
Inert Electrodes
These electrodes do not participate in the electrolysis reaction but help to transfer electrons from the cathode to the anode. Platinum (Pt) and gold are examples of such types of electrodes.
When copper sulfate (CuSO4) is electrolyzed between the platinum electrode, metallic copper is deposited at the cathode by receiving two electrons, and hydroxyl ion (OH−) is deposited at the anode.
Due to high deposition potential, sulfate ions (SO4−2) will not be discharged at the anode.
Reactive Electrodes
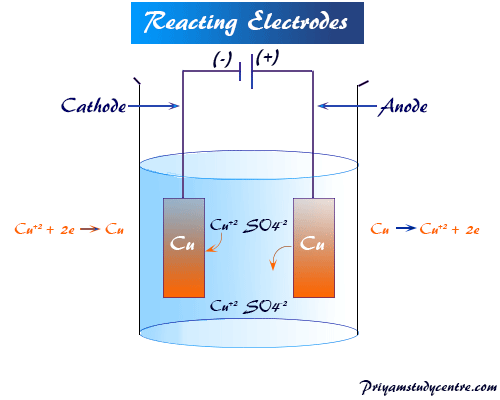
They participate in the electrolysis reactions either by contributing ions to the solution or accepting the discharged ions from the solution.
When copper sulfate solution is electrolyzed between two copper electrodes, copper is deposited at the cathode as usual but equivalent copper is dissolved. The anode and the process are used for the purification of pure copper from its impure form.
Electrode in Electrochemical Cell
Electrochemical cells are devices that generate electrical energy from the chemical reaction of the cell or use electrical energy to perform the chemical reaction.
The electrochemical cell is mainly of two types,
- Primary cell
- Secondary cell
What is Primary Cell?
A primary cell is a device where an electrode like a cathode and anode is fixed and the cell reaction cannot be reversed.
- When the cell is charged, the anode becomes positive and the cathode becomes positive.
- When discharged it works like a primary cell where the anode is negative and the cathode is the positive electrode.
What is Secondary Cell?
A secondary cell like a rechargeable battery is a chemical cell where chemical reactions are reversible. The lithium ion batteries are examples of secondary rechargeable chemical cells.
Uses of Electrodes
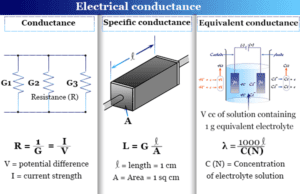
- Different types of electrodes like gold, platinum, copper, and silver are used to define electrical conductivity during electrolysis.
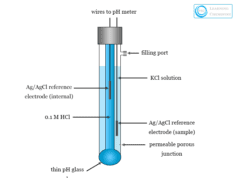
- A glass electrode is used for the measurement of the pH scale of the solution.
- Batteries are devices that contain a variety of electrodes depending on the type. For example, lead-acid batteries contain lead electrodes, zinc-carbon batteries contain zinc and carbon electrodes, and lithium polymer batteries contain solid lithium polymer electrodes.
- The electrode is also used in welding, membrane cathode, ECG, ECT, EEG, and defibrillator in biochemical research.