Electrolysis Definition in Chemistry
Electrolysis in chemistry is defined as a process where chemical changes occur at the electrodes (cathode or anode) due to the passage of electricity through the electrolytic solution. From definition, the process of electrolysis in chemistry work through oxidation reduction or redox reaction by which the substance loses or gains electrons. Therefore, the electrolysis of water is a process in which electricity is used to split water into hydrogen and oxygen. Applications of the electrolysis process are commercially important for the extraction, electrolytic refining, and electroplating of metals like copper, nickel, silver, and gold by using electrolytic cells. Faraday’s law in chemistry derives the quantitative formula between electricity and chemical change that occurs during electrolysis.
Acids, bases, and salts in the state of solution or a fused state conduct electricity and they are called electrolytes. The current in electrolytic solution usually enters or leaves through some rods or strips of metal called electrodes. The one through which current enters is called anode or positive electrode. However, the one through which the current leaves is called a cathode or negative electrode.
The term electrolysis was first introduced in the 19th century by English scientist Michael Faraday. Today, the applications of the electrolysis process are commercially important because such process is used widely in separating or obtaining pure metals from naturally occurring ores.
Electrolysis Process
An electrolysis reaction may take place in a unit called an electrolyzer. Like fuel cells, electrolyzers contain an anode and a cathode separated by an electrolyte solution.
An electrolyte, electrodes, electrolyzer, and an external DC power source are required to perform electrolysis. A partition like an ion exchange resin membrane or a salt bridge is also needed to separate the products.
- A block or strip of metal, graphite, or semiconductor materials is used as an anode or a cathode.
- A solution of metal salt, acids, and alkalies is used as an electrolyte during the electrolysis process.
- When a direct electric current is passed, a reduction reaction occurs at the cathode.
- An oxidation reaction also occurs at the anode during electrolysis.
Therefore, the process of electrolysis work by interchanging atoms or ions by the addition or removal of electrons due to applied electrode potential.
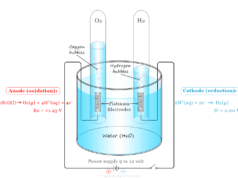
Electrolysis of Water
Pure water is a very poor conductor of electricity and the dissociation of water molecules into H+ and OH− ions is negligible. However, the addition of a small amount of electrolyte increses the conductivity of water to a considerable extent. The electrolysis of water can be work out in an apparatus is given below in the diagram:
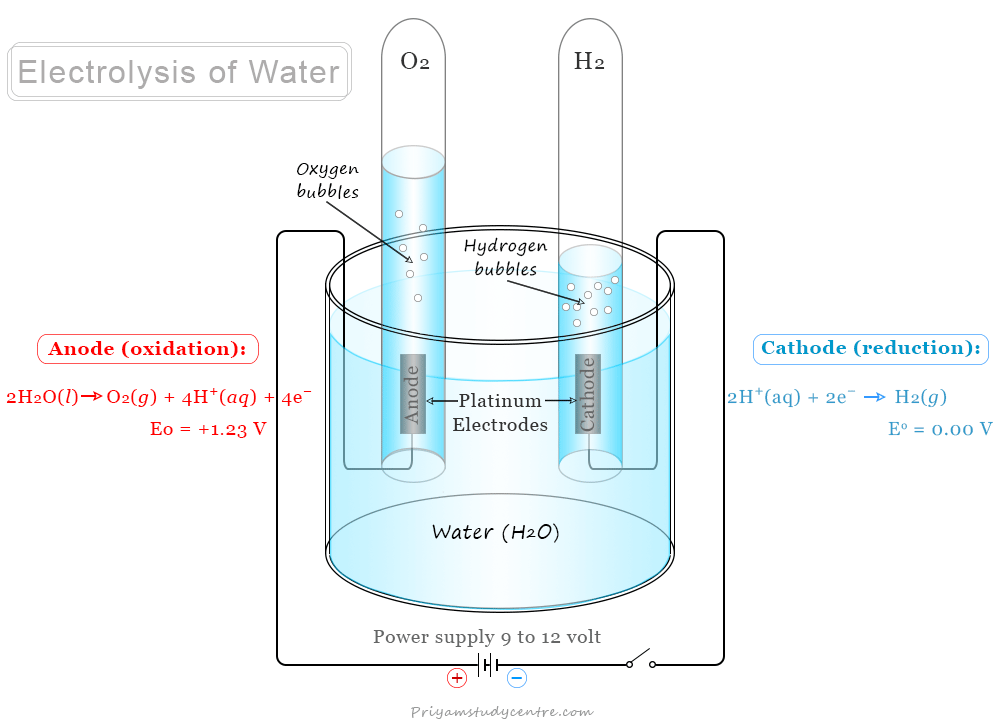
The apparatus contains a glass vessel having two vertical platinum foils. The water is acidulated by adding a few drops of concentrated sulfuric acid.
When platinum foils are connected to DC current, the bubbles of gases begin to evolve from the surface of the platinum foil. On passing direct current, water molecules dissociate to form H+ and OH− ions.
H2O → H+ + OH−
Cathode Reaction
When electrolysis work, H+ ions migrate to the cathode and accept an electron to form a hydrogen atom. Therefore, a reduction reaction occurs at the cathode. The hydrogen atoms then combine to form molecular hydrogen at the cathode.
H+ + e− → H (Reduction)
H + H → H2 (g)
Anode Reaction
Similarly, OH− migrates toward the anode and releases an electron to form an OH radical. Therefore, an oxidation reaction occurs at the anode. The OH radicals finally converted to oxygen molecules at the anode.
OH− → OH + e− (Oxidation)
4OH → H2O + O2 (g)
Therefore, electrolysis of water in chemistry is defined as a process where electricity is used to split water into hydrogen (H2) and oxygen (O2) molecules. The volume of oxygen collected at the anode is to be half of the hydrogen.
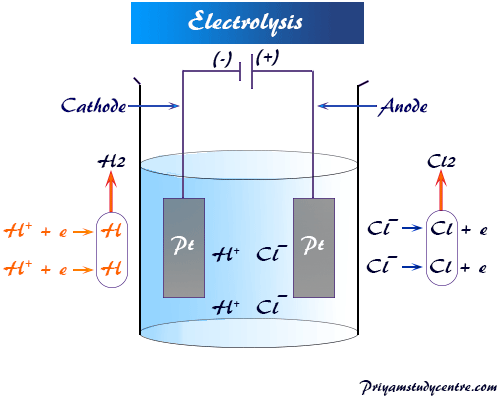
Electrolysis of Hydrochloric Acid
The process of electrolysis and the mechanism of electric conductance of hydrochloric acid solution define that there is a surplus of electrons at the cathode and a deficit of electrons at the anode.
- The hydrogen ion (H+) in solution moves toward the cathode by Coulombic force of attraction and receives one electron to form the hydrogen atom.
- Similarly, chlorine ion from the solution moves towards the anode and departs one electron to form a neutral chlorine atom.
One electron transfers from the cathode to the anode with the help of hydrochloric acid solutions or medium, and due to the conductance of electric current or electrolysis, the ions are decomposed at the electrode.
Two hydrogen atoms form hydrogen gas and two chlorine atoms form chlorine molecules, escape from the electrolytic solution.
Electrolysis and Electrolytic Cell
Electrolytic cells require an external power source for electrolysis reactions, while electrochemical cells can generate electrical energy from spontaneous oxidation-reduction or redox reactions. An electrolyte cell contains two electrodes held apart from each other and dipped in a fused or dissolved electrolytic solution. A direct current provides free energy to drive cell reaction in a non-spontaneous direction when electrolysis work in an electrolytic cell.
Electrolysis in electrolytic cells has many industrial applications such as electroplating, electrolytic refining metals, and production of many inorganic and organic compounds. The electrolysis of sodium hydroxide and potassium hydroxide by English chemist Sir Humphrey Davey in 1808 led to the discovery of these two metallic elements sodium and potassium.
Electrolysis in Water
When certain aqueous solutions are electrolyzed, water is involved in electrode reactions rather than ions derived from the solute. Therefore, the current-carrying ions are not necessary to discharge at the two electrodes (cathode and anode). The electrolysis reactions of some aqueous salt solutions are given below in the table,
| Solution | Electrodes | Cathodic reaction | Anodic reaction |
| NaCl | Inert | 2H2O + 2e− → H2 (g) + 2OH− | 2Cl− → Cl2 (g) + 2e− |
| Na2SO4 | Inert | 2H2O + 2e− → H2 (g) + 2OH− | H2O → ½O2 (g) + 2H+ + 2e− |
| CuSO4 | Inert | Cu+2 + 2e− → Cu | H2O → ½O2 (g) + 2H+ + 2e− |
| CuCl2 | Inert | Cu+2 + 2e− → Cu | 2Cl− → Cl2 (g) + 2e− |
| CuSO4 | Copper | Cu+2 + 2e− → Cu | Cu → Cu+2 + 2e− |
Electrolysis of Copper Sulphate Using Platinum Electrodes
A solution of copper sulfate in water taken in an electrolyzer contains two platinum electrodes.
CuSO4 → Cu+2 + SO4−2
H2O → H+ + OH−
The changes occur in the cathode and anode during the DC current flow. A reduction reaction takes place at the cathode when electrolysis work while an oxidation reaction takes place at the anode.
Among Cu2+ and H+, Cu+2 will be reduced at the cathode because Cu+2/Cu has a higher standard reduction potential. Therefore, metallic copper can be deposited at the cathode.
Cu+2 (aq) + 2e− → Cu (s)
Similarly, among SO4−2 and OH−, OH− will be oxidized at the anode because OH− has a greater tendency to get oxidized. Therefore, oxygen gas will released at the anode.
H2O → ½O2 + 2H+ + 2e−
Electrolysis of Copper Sulphate Using Copper Electrodes
Electrolysis of copper sulfate using copper electrodes is used commonly in the electrorefining of copper. A solution of copper sulfate in water taken in an electrolyzer contains two copper electrodes.
CuSO4 → Cu+2 + SO4−2
H2O → H+ + OH−
On passing the direct electric current, both H+ and Cu+2 ions migrated toward the cathode. Among these two ions, Cu+2 will be reduced at the cathode because Cu+2/Cu has a higher standard reduction potential.
Cu+2 (aq) + 2e− → Cu (s)
When the electrolysis work, each copper atom of anode gives up two electrons and forms Cu+2 ions. Therefore, the anode gets oxidized to form copper ions and goes to the electrolyte solution.
Cu (s) → Cu+2 (aq) + 2e−
Faraday’s Law of Electrolysis
During electrolysis, Faraday’s law in chemistry derives the quantitative formula between electricity and the amount of decomposition in an electrode or the weight of different substances decomposed at the different electrodes.
Faraday’s First Law of Electrolysis
According to Faraday’s first law of electrolysis, the amount of decomposition at the electrode (w) is directly proportional to the quantity of electric current or electricity (Q) passed.
The mathematical formula of the first law,
w = ZQ
Where w = electrochemical equivalence of decomposing substances.
Again, w = ZIt
Where I = current strength or amount of electricity passing in unit time
t = time of flow of electricity
Z = electrochemical equivalent
Faraday’s Second Law of Electrolysis
Faraday’s second law of electrolysis states that the weight of different substances decomposed at the different electrodes by given electricity is proportional to their equivalent weight.
The mathematical formula of the second law of electrolysis,
w1/w2 = E1/E2
Where w1 and w2 = weight of a decomposed substance
E1 and E2 = equivalent weights
1 Faraday of Electricity
One Faraday defines the amount of electricity required to decompose one gram equivalent electrolytic substance in chemistry or chemical science.
The numerical value of 1F = 96496 coulombs
≈ 96500 coulombs.
The electrochemical equivalent of silver (atomic mass = 108),
ZAg = 108/96500
= 0.001118 gm/coulomb
This law was used to construct the instrument Coulometer to determine the amount of electricity passing through the circuit by determining the quantity of a substance that decomposes at an electrode in an electrolysis solution.
Avogadro Number Calculation
In learning chemistry, Faraday’s second law helps to determine the Avogadro number (N0). For the decomposition of a 1-mole substance at the electrode, ZF electricity is required. Similarly, for 1-mole substance ZeN0 amount of charge.
ZeN0 = ZF
or N0 = F/e
The numerical value of Avogadro number
N0 = 96500/1.6 × 10−19
= 6.022 × 1023
Applications of Electrolysis
Today, the applications of the electrolysis process are commercially important because such process is used widely in separating or extracting pure metals and non-metals from naturally occurring ores. The electroplating by electrolysis process is also used to protect copper, iron, and aluminum against atmospheric corrosion.
Refining of Metals
The purification of metals by the electrolysis process has important industrial applications. It is used for refining metals like copper, zinc, nickel, silver, and gold from their impure form.
- A thick block of impure metal is used as an anode and a thin strip of pure metal is used as a cathode.
- A solution of metal salt is used as an electrolyte during electrolytic refining.
- When DC current is passed, metal ions from the electrolyte are reduced and deposited at the cathode.
- An equivalent amount of impure metal from the anode gets oxidized to form metal ions and goes to the electrolyte solution.
The process is repeated until the whole of the metal ion from the impure block is dissolved and deposited. The soluble impurities go into the solution while insoluble impurities are settled down as anode mud below the anode.
| Refining Metal | Electrolyte | Anode | Cathode |
| Copper | Aqueous solution of copper sulfate (CuSO4) and sulfuric acid (H2SO4) | Impure copper | Pure copper |
| Zinc | Zinc sulfate (ZnSO4) solution | Impure zinc | Pure zinc |
| Nickel | Solution of nickel sulfate or nickel chloride | Impure nickel | Pure nickel |
| Silver | Aqueous solution of silver nitrate (AgNO3) | Impure silver | Pure silver |
| Gold | Solution of hydrochloric acid and gold chloride | Impure gold | Pure gold |
Extraction of Metals
The extraction of metals by electrolysis process has also important industrial applications. It is used for extracting metals like aluminum, lithium, sodium, and magnesium from their ores or compounds. The non-metal like fluorine, and chlorine may also extracted industrially by electrolysis.
During extraction, the chloride salts of metals in the molten form are used for electrolysis. Metal is deposited at the cathode and chlorine gas is liberated at the anode. For example, when the molten sodium chloride is electrolyzed, sodium is deposited at the cathode, and chlorine gas is liberated at the anode.
At cathode: Na+ + e− → Na (reduction)
At anode: 2Cl− → Cl2 + 2e−
Therefore, molten sodium chloride produced metallic sodium and chlorine gas.
Similarly, aluminum is obtained by electrolytic reduction of molten aluminum oxide or alumina. Alumina is dissolved in molten synthetic cryolite (Na3AlF6) and some fluorspar (CaF2) to lower its melting point to electrolyzed.
At cathode: 2Al+3 + 6e− → 2Al (reduction)
At anode: 3O−2 → 3/2O2 + 6e− (oxidation)
Electroplating of Metals
Electrolysis is a process that is used widely for coating or electroplating metals. Electroplating is commonly used to protect corrosive metals such as copper, iron, and aluminum from atmospheric corrosion. For better looks, superior metals like gold, silver, chromium, and nickel are coated with copper, iron, and aluminum by electrolysis.
- The metal that is electroplated is used as a cathode.
- A solution of electroplating metal salt is used as an electrolyte during electroplating.
- A rod or plate of pure electroplating metal is used as an anode.
- When DC current is passed, electroplating metal ions from the electrolyte are reduced and produce a thin layer on the cathode.
- An equivalent amount of pure electroplating metal from the anode gets oxidized to form metal ions and goes to the electrolyte solution.
Tin and chromium metals are resistant to corrosion. Therefore, a thin layer of tin or chromium is used on iron or steel objects to protect them from rusting.
| Plating metal | Electrolyte | Anode |
| Silver | Sodium argentocyanide, Na[Ag(CN)2] solution | Pure silver |
| Gold | Solution of potassium gold cyanide, K[Au(CN)2] | Pure gold |
| Chromium | Solution of chromic acid (H2CrO4) and sulfuric acid (H2SO4) | Pure chromium |
| Nickel | Solution of nickel sulfate (NiSO4) | Pure nickel |
Other Applications
- The electrolysis of the aqueous solution of sodium chloride produced sodium hydroxide and chlorine gas.
- The process of electrolysis is used for the production of hydrogen and oxygen from water solutions.
- The electrolysis formula uses fuel cells, which work by producing chemical energy by combustion of fuels like hydrogen, carbon monoxide, methane, propane, and methyl alcohol.
- It is directly converted into electrical energy used for a pollution-free continuous source of energy in spacecraft and nuclear submarines.
Frequently Asked Questions
What is Electrolysis in Chemistry?
Electrolysis in chemistry is defined as a process or technique where chemical changes occur at the electrodes (cathode or anode) due to the passage of direct current (DC) through the electrolytic solution. An electrolysis reaction may take place in a unit called an electrolyzer. Like fuel cells, electrolyzers also contain two electrodes (anode and cathode) separated by an electrolyte solution.
The electrolysis of molten salt produces ions which are present in the salt. However, When certain aqueous solutions are electrolyzed, water is involved in electrode reactions rather than ions derived from the solute. Therefore, the current-carrying ions are not necessary to discharge at the two electrodes (cathode and anode).
What is Electrolysis of Water?
The electrolysis of water in chemistry is defined as a process where electricity is used to split water into hydrogen (H2) and oxygen (O2) molecules. The volume of oxygen collected at the anode is to be half of the hydrogen.
The production of pure hydrogen and oxygen is the main application of the electrolysis of water. Generally, water electrolysis equipment can be used to generate oxygen for the International Space Station (ISS), production of hydrogen for the chemical industry and renewable energy generation, production of deuterium from heavy water, etc.
How does Electrolysis Work?
The electrolysis work by passing an electric current through an electrolytic solution (fused or dissolved state). Chemical changes occur at the electrodes (cathode or anode) due to the passage of direct current (DC) through the electrolytic solution.
The process of electrolysis work by interchanging atoms or ions by the addition or removal of electrons due to applied electrode potential. An electrolyte, electrodes, an electrolyzer, a partition (salt bridge), and an external DC power source are required to perform any type of electrolysis.
Is Electrolysis a Chemical Change?
Yes, electrolysis is a chemical change because a chemical reaction occurs at the two electrodes (cathode and anode) during the electrolysis process.