Acids and Bases Properties
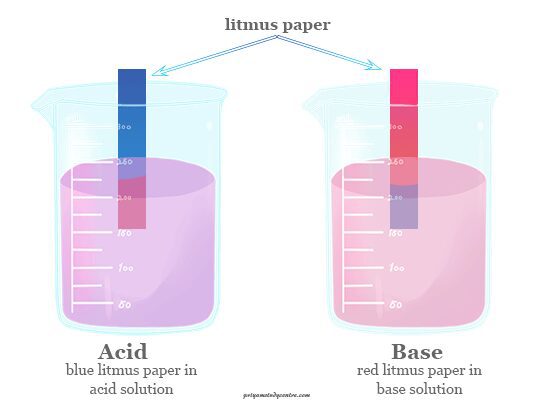
Acids and bases in chemistry commonly possess some opposite properties. For example, acids are chemical substances whose aqueous or water solution is sour in nature and properties to turn blue litmus to red. Bases are substances whose aqueous solution is slippery, bitter in taste, and properties to change colour of red litmus into the blue. A strong acid molecule can react with the strong base molecule to form a neutral salt solution. Modern ideas about acid-base theory based on electronic configuration, it is very useful for describing the acid-base properties. Five theories are very useful in describing the properties and neutralization reactions of acids and bases. These theories are Arrhenius theory, Lewis acid-base theory, Bronsted Lowry conjugate acid-base pair, Hard soft acid base theory, and pH scale. Due to some limitations, we can not explain the acids and bases definition, properties, and neutralization reaction by only one concept or theory.
Arrhenius Acid and Base
In learning chemistry, Arrhenius acid and base definition suggested that acid dissociates hydrogen ions whereas base hydroxyl ions in water or aqueous solution.
Arrhenius’s theory can explain the acid and base properties in water solution only. Therefore, sulfuric acid, nitric acid, and hydrogen chloride molecules show acid properties only when it is dissolved in a water solution. If we use organic solutions like benzene or alcohol or gases, these substances do not show acid properties according to Arrhenius.
Arrhenius Acid Base Theory
Arrhenius defines acid base properties in water solution only.
- According to his definitions, an acid when dissolved in water, dissociates hydrogen ions and anions.
- Base when dissolved in water dissociates into hydroxyl ions and cations.
Acids bases neutralization reaction has properties to the formation of the water molecules.
HCl → H+ + Cl−
NaOH → Na+ + OH−
H+ + OH− ⇆ H2O
Acid Base Neutralization Reaction
Acids and bases reactions in chemistry are highly useful in explaining the acid base neutralization properties in solution. When a strong acid HA reacts with a strong base BOH in the water solution, all are completely dissociating.
H+ + A− + B+ + OH− ⇆ B+ + A− + H2O
When the above equation balancing by likes ions,
H+ + OH− ⇆ H2O
Limitations of Arrhenius Theory
- According to Arrhenius’s definitions, HCl is regarded as an acid when dissolved in water. But if we use an organic solvent like ethyl alcohol or acetic acid or gases, HCl is not considered an acid.
- Arrhenius’s limitations cannot account for the properties of acids bases reactions in non-aqueous solvents. For example, NH4NO3 or boric acid in liquid ammonia is an acid, though it does not give H+ ions.
- The neutralization examples of acid-base are limited to those reactions which can occur in aqueous solutions only. Therefore, the redox reaction where oxidation number changes by loss or gain by electron can not be explained by this theory.
- Arrhenius theory cannot explain the acid properties of metallic elements present in the periodic table.
Acids and Bases Equilibrium
The heat of neutralization is the heat change associated with the neutralization of 1 g equivalent acid by alkali in their very dilute solution. Dilute concentration is used in order to avoid any heat change due to the mixing of acid and base.
Neutralization is a chemical equilibrium reaction or exothermic reaction. The heat of neutralization of strong acid and strong base is found to be a constant, equal to 13.6 kcal at 25 °C temperature.
At equilibrium, all strong acids and bases properties to the formation of 1 mole water (molecular weight of water = 18 gm mol−1) and thermodynamics enthalpy change (ΔH) will also be the same.
Therefore, the specific heat release during neutralization of HCl, HClO4, HNO3, HBr, HI, and H2SO4 and strong base NaOH, KOH, RbOH, and Ca(OH)2), namely 13.6 kcal/mole or 56 KJ/mole.
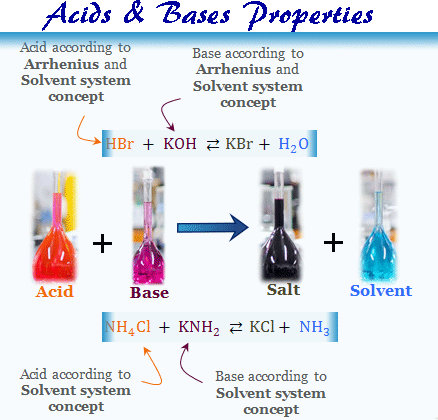
Solvent System Concept
The solvent system concept is the extension of the Arrhenius water-ion system. The acids and bases properties of ammonia, hydrogen fluoride, hydrogen peroxide, acetic acid, and many organic compounds in the aqueous or nonaqueous solution can easily be explained by this theory.
- According to the solvent system concept, an acid is a substance that dissociation in solution to form the same cation as does the solvent itself due to auto-ionization.
- A base is one that, gives dissociation in solution to form the same anion as does the solvent itself on its auto-ionization.
Dissociation of Acids and Bases in Water
Water solution has both acidic and basic properties and it dissociates H+ and OH− ions. But H+ ion is readily polarization by other anions or molecules that exist in the solution.
H2O + H2O → H3O + OH−
According to the solvent system concept, all those substances that have properties to give H3O+ ions in H2O will act as acids, and all those substances that can give OH− ions in H2O will act as bases.
Acid Base Reaction in Liquid Ammonia
Ammonia solution dissociates into two oppositely charged ions like NH4+ and NH2−.
NH3 + NH3 → NH4+ + NH2−
Therefore, all those substances that have properties to give NH4+ ions in liquid ammonia will act as acids. But all those substances which can give NH2− ions in liquid ammonia have properties of bases.
Neutralization Reaction Examples
Acid behavior is not confined to the solution containing hydrogen ions only. Even in non-protonic solvents acid-base neutralization reaction also is performed. Therefore, acid-base phenomena or neutralization reactions depend on the solvent.
Some examples of acid-base neutralization reactions are given below the chart,
| Solvent | Acid | Base | Salt |
| water (H2O) | H3O+ (HBr) | OH− (KOH) | KBr |
| ammonia (NH3) | NH4+ (NH4Br) | NH2− (KNH2) | KBr |
| ethanol (C2H5OH) | C2H5OH2+ (HBr) | C2H5O− (KOC2H5) | KBr |
| sulfur dioxide (SO2) | SO+2 (SOBr2) | SO3−2 (K2SO3) | KBr |
NH4Cl + KNH2 ⇆ KCl + NH3
SOBr2 + K2SO3 ⇆ 2KBr + 2SO2
NOCl + AgNO3 ⇆ AgCl + N2O4
HBr + KOH ⇆ KBr + H2O
Limitations of Solvent System Concept
- Solvent system theory does not consider a number of acid-base reactions included in the protonic definition.
- It limits acid-base phenomena to the solvent systems only. Therefore, it does not explain the acid-base reactions that may occur in the absence of solvent.
- It can not explain the neutralization reactions of acids and bases occurring without the presence of ions.